Five biochemical varieties of Alzheimer’s disease have been uncovered by Dutch researchers, each of which may require a different treatment. As a result, previously studied medications may look ineffective or only marginally effective. This is the conclusion of Alzheimer Center Amsterdam researcher Betty Tijms and colleagues from Amsterdam UMC and Maastricht University.
Amyloid and tau proteins aggregate in the brains of people with Alzheimer’s disease, but their underlying biological mechanisms are largely unclear.
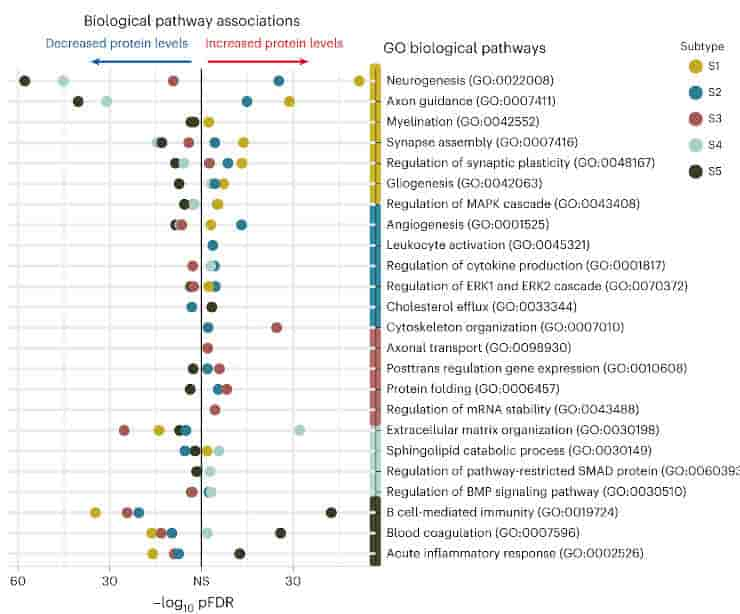
Many different pathophysiological processes are associated with amyloid and tau pathology, according to genetic, brain tissue proteomics, and gene expression studies. Theses processes include but are not limited to synaptic plasticity, the innate immune system, neuroinflammation, lipid metabolism, RNA metabolism, the matrisome, and vascular function.
Researchers used new mass spectrometry-based proteomic analysis methods to investigate these other processes in the cerebrospinal fluid of people who had amyloid and tau clumps.
Subgroups of Alzheimer’s Disease
Betty Tijms and Pieter Jelle Visser looked at 1,058 proteins in the cerebral fluid of 419 Alzheimer’s patients. Within this group, they discovered five biological variations.
The first type is distinguished by a rise in amyloid formation. The blood-brain barrier is compromised in the second type, resulting in decreased amyloid production and nerve cell development.
Furthermore, the variants differ in terms of protein synthesis, immune system function, and the function of the choroid plexus, the organ that creates cerebrospinal fluid. Patients with distinct Alzheimer’s variations differed in other elements of the disease as well.
For example, the researchers found a faster course of the disease in certain subgroups.
Therapeutic Implications
The findings have significant implications for drug research. They could imply that a certain medicine is exclusively effective in one type of Alzheimer’s disease.
Given the diverse patterns of molecular processes and Alzheimer’s disease genetic risk profiles, specialized treatments for Alzheimer’s subtypes are expected. Individuals with subtype 1 may benefit from TREM2-activating treatments, those with subtype 2 from innate immune inhibitors, those with subtype 3 from antisense oligonucleotides that restore RNA processing, those with subtype 4 from monocyte infiltration inhibition, and those with subtype 5 from cerebrovascular treatments.
At the same time, side effects from specific medications may vary depending on subtype. For example, while antibodies may pass the blood-brain barrier more easily in subtype 5, these individuals may be more vulnerable to the cerebral hemorrhage that can occur with antibody treatment.
A logical next step for the research team would be to demonstrate that the Alzheimer’s variants do indeed react differently to medicines in order to treat all patients with appropriate medicines in the future.
Abstract
Alzheimer’s disease (AD) is heterogenous at the molecular level. Understanding this heterogeneity is critical for AD drug development. Here we define AD molecular subtypes using mass spectrometry proteomics in cerebrospinal fluid, based on 1,058 proteins, with different levels in individuals with AD (n = 419) compared to controls (n = 187). These AD subtypes had alterations in protein levels that were associated with distinct molecular processes: subtype 1 was characterized by proteins related to neuronal hyperplasticity; subtype 2 by innate immune activation; subtype 3 by RNA dysregulation; subtype 4 by choroid plexus dysfunction; and subtype 5 by blood–brain barrier impairment. Each subtype was related to specific AD genetic risk variants, for example, subtype 1 was enriched with TREM2 R47H. Subtypes also differed in clinical outcomes, survival times and anatomical patterns of brain atrophy. These results indicate molecular heterogeneity in AD and highlight the need for personalized medicine.
Reference:
- Tijms, B.M., Vromen, E.M., Mjaavatten, O. et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat Aging (2024). Doi: 10.1038/s43587-023-00550-7
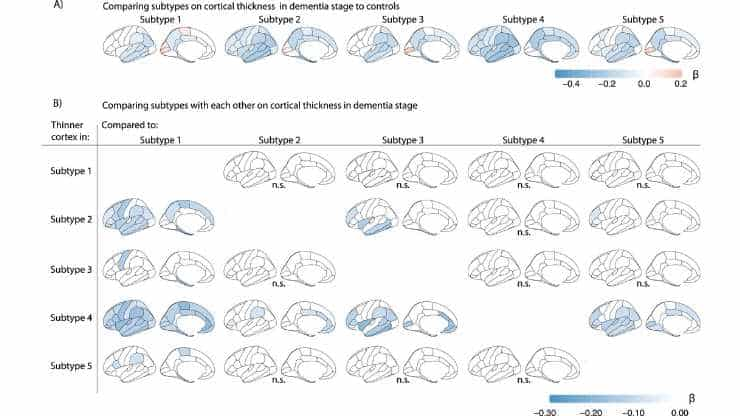
Image: Comparing subtypes on cortical thickness in dementia stage. ß indicates mean cortical thickness in mm, averaged over right and left hemispheres and adjusted for age and sex. (a) Cortical thickness compared between AD subtypes (in dementia stage) with controls. (b) Cortical thickness comparisons between AD subtypes within the dementia stage. Credit: Nat Aging (2024). Doi: 10.1038/s43587-023-00550-7