The majority of neurodegenerative diseases, such as dementias, are caused by proteins that aggregate into filaments known as amyloids. Researchers may now target these proteins for diagnostic testing and treatment because they have discovered the proteins that aggregate in the majority of these disorders.
However, scientists had yet to discover the rogue protein in approximately 10% of cases of frontotemporal dementia. In these situations, research led by scientists from the Medical Research Council (MRC) Laboratory of Molecular Biology, in Cambridge has now identified aggregated forms of the protein TAF15.
“This discovery transforms our understanding of the molecular basis of frontotemporal dementia. It is a rare finding of a new member of the small group of proteins known to aggregate in neurodegenerative disease,”
said Dr. Benjamin Ryskeldi-Falcon, who led the study at the MRC Laboratory of Molecular Biology.
Targeting Protein Aggregates
Frontotemporal dementia is caused by degeneration of the brain’s frontal and temporal lobes, which control emotions, personality, and conduct, as well as speech and word understanding.
It typically begins at a younger age than Alzheimer’s disease, with most cases identified in persons aged 45 to 65, however it can affect people of any age.
“Now that we have identified the key protein and its structure, we can start to target it for the diagnosis and therapy of this type of frontotemporal dementia, similar to strategies already in the pipeline for targeting the aggregates of amyloid-beta and tau proteins that characterize Alzheimer’s disease,”
Ryskeldi-Falcon said.
The scientists used cutting-edge cryo-electron microscopy (cryo-EM) to study protein aggregates from the brains of four people who had this type of frontotemporal dementia at atomic resolution. The University College London Queen Square Institute of Neurology’s Tammaryn Lashley and Indiana University School of Medicine’s Bernardino Ghetti identified the donated brains.
TATA-binding Protein-associated Factor 15
Based on parallels with other neurodegenerative diseases, scientists had long assumed that a protein called FUS aggregated in this form of dementia.
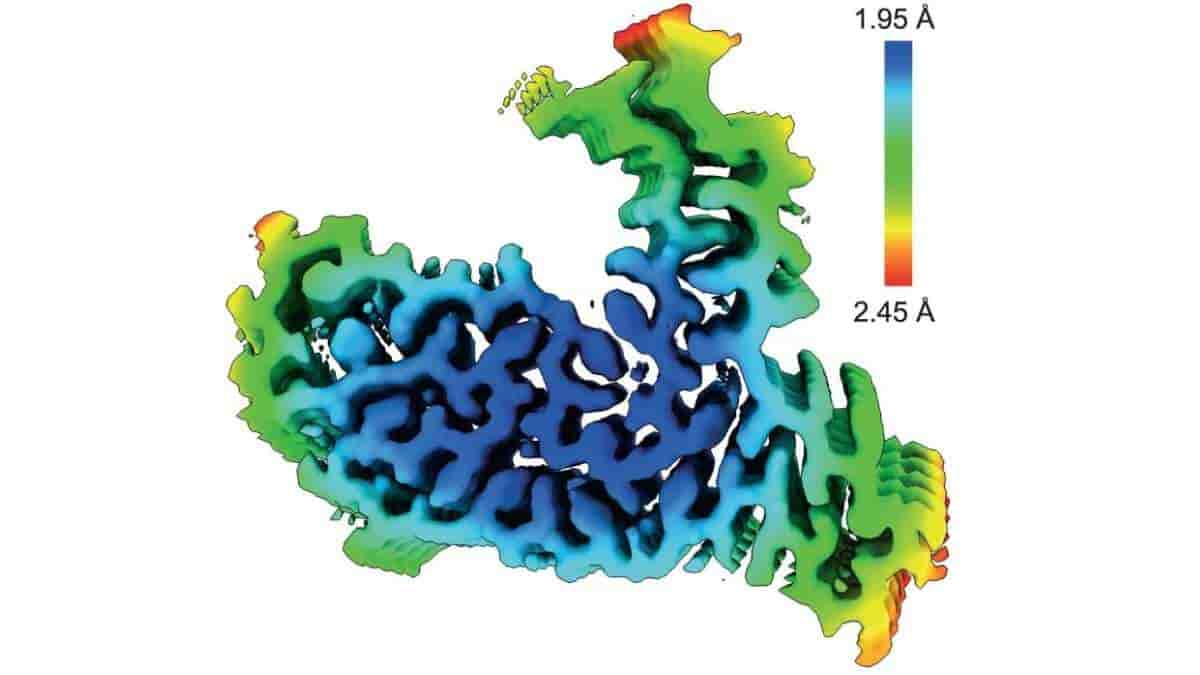
The researchers at the MRC Laboratory of Molecular Biology were able to determine that the protein aggregates from each brain had the same atomic structure using cryo-EM. Surprisingly, the protein in question was not FUS, but rather TATA-binding protein-associated factor 15 (TAF15).
“This is an unexpected result because, before this study, TAF15 was not known to form amyloid filaments in neurodegenerative diseases and no structures of the protein existed. Cryo-EM is transforming our understanding of the molecular pathology of dementia and neurodegenerative diseases more broadly by giving us insights that were beyond the capabilities of previous technologies,”
said first author Dr. Stephan Tetter, also from the MRC Laboratory of Molecular Biology.
“The technical challenge of performing cryo-EM meant that we were only able to look at the brains of four individuals. However, now that we know the key protein and its structure, we have the potential to develop tools to screen for these abnormal protein aggregates in hundreds of patient samples to test how widespread they are,”
Dr. Ryskeldi-Falcon added.
Motor Neuron Disease
A condition known as motor neuron disease, in which people gradually lose control over their muscles, is also present in some frontotemporal dementia patients.
In this study, two of the individuals who donated their brains had signs of both diseases. For these individuals, the researchers identified the same aggregated structure of TAF15 in brain regions associated with motor neuron disease.
“The presence of the same TAF15 aggregates in two individuals who had frontotemporal dementia and signs of motor neuron disease raises the possibility that TAF15 may contribute to both diseases. We are now studying whether aberrant aggregated TAF15 is present in people who have motor neuron disease in the absence of frontotemporal dementia,”
Dr. Ryskeldi-Falcon said.
“This latest study identifying the protein linked to a type of frontotemporal dementia continues the MRC LMB’s success in elucidating dementia-related protein structures by cryoEM, which includes the first structure for the key dementia protein tau. Knowing the identity and basic structure of these filaments in this rare form of early-onset dementia is vital to developing early diagnostic tests and drugs to combat their formation,”
Dr. Charlotte Durkin said. Dr. Durkin is head of the Medical Research Council’s Molecular and Cellular Medicine Board.
The work was supported by the electron microscopy and scientific computing facilities at the MRC Laboratory of Molecular Biology and by the Center for Medical Genomics at the Indiana University School of Medicine, by the Medical Research Council as part of United Kingdom Research and Innovation (also known as UK Research and Innovation); the US National Institutes of Health; the Alzheimer’s Society; the Association for Frontotemporal Degeneration; a Swiss National Science Foundation Postdoctoral Fellowship; and a Leverhulme Early Career Fellowship.
Abstract
Frontotemporal lobar degeneration (FTLD) causes frontotemporal dementia (FTD), the most common form of dementia after Alzheimer’s disease, and is often also associated with motor disorders. The pathological hallmarks of FTLD are neuronal inclusions of specific, abnormally assembled proteins. In the majority of cases the inclusions contain amyloid filament assemblies of TAR DNA-binding protein 43 (TDP-43) or tau, with distinct filament structures characterizing different FTLD subtypes. The presence of amyloid filaments and their identities and structures in the remaining approximately 10% of FTLD cases are unknown but are widely believed to be composed of the protein fused in sarcoma (FUS, also known as translocated in liposarcoma). As such, these cases are commonly referred to as FTLD–FUS. Here we used cryogenic electron microscopy (cryo-EM) to determine the structures of amyloid filaments extracted from the prefrontal and temporal cortices of four individuals with FTLD–FUS. Surprisingly, we found abundant amyloid filaments of the FUS homologue TATA-binding protein-associated factor 15 (TAF15, also known as TATA-binding protein-associated factor 2N) rather than of FUS itself. The filament fold is formed from residues 7–99 in the low-complexity domain (LCD) of TAF15 and was identical between individuals. Furthermore, we found TAF15 filaments with the same fold in the motor cortex and brainstem of two of the individuals, both showing upper and lower motor neuron pathology. The formation of TAF15 amyloid filaments with a characteristic fold in FTLD establishes TAF15 proteinopathy in neurodegenerative disease. The structure of TAF15 amyloid filaments provides a basis for the development of model systems of neurodegenerative disease, as well as for the design of diagnostic and therapeutic tools targeting TAF15 proteinopathy.
Reference:
- Tetter, S., Arseni, D., Murzin, A.G. et al. TAF15 amyloid filaments in frontotemporal lobar degeneration. Nature (2023). Doi: 10.1038/s41586-023-06801-2
Top Image: TAF15 amyloid filaments from FTLD-FET, with rainbow-coloured local resolution estimates viewed perpendicular to the helical axis. Credit: Nature (2023). Doi: 10.1038/s41586-023-06801-2