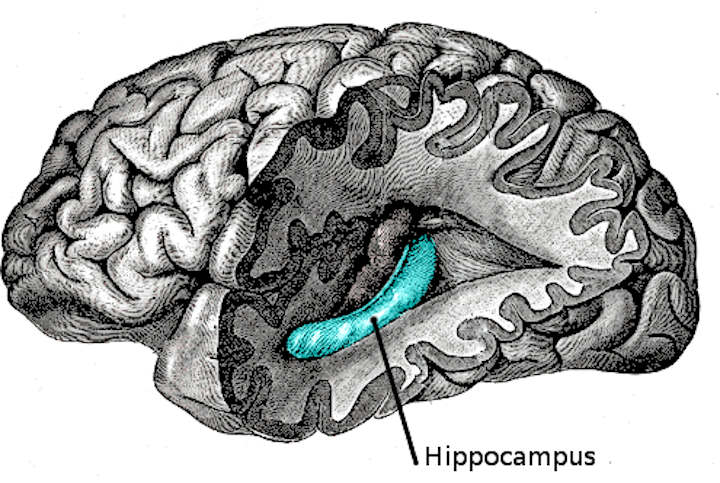
The hippocampus (named after its resemblance to the seahorse, from the Greek ἱππόκαμπος, “seahorse” from ἵππος hippos, “horse” and κάμπος kampos, “sea monster”) is a major component of the brains of humans and other vertebrates.
Humans and other mammals have two hippocampi, one in each side of the brain. It belongs to the limbic system and plays important roles in the consolidation of information from short-term memory to long-term memory and spatial navigation. The hippocampus is located under the cerebral cortex; and in primates it is located in the medial temporal lobe, underneath the cortical surface. It contains two main interlocking parts: Ammon’s horn and the dentate gyrus.
In Alzheimer’s disease, the hippocampus is one of the first regions of the brain to suffer damage; memory loss and disorientation are included among the early symptoms. Damage to the hippocampus can also result from oxygen starvation (hypoxia), encephalitis, or medial temporal lobe epilepsy. People with extensive, bilateral hippocampal damage may experience anterograde amnesia—the inability to form or retain new memories.
In rodents, the hippocampus has been studied extensively as part of a brain system responsible for spatial memory and navigation. Many neurons in the rat and mouse hippocampus respond as place cells: that is, they fire bursts of action potentials when the animal passes through a specific part of its environment. Hippocampal place cells interact extensively with head direction cells, whose activity acts as an inertial compass, and conjecturally with grid cells in the neighboring entorhinal cortex.
Since different neuronal cell types are neatly organized into layers in the hippocampus, it has frequently been used as a model system for studying neurophysiology. The form of neuroplasticity known as long-term potentiation (LTP) was first discovered to occur in the hippocampus and has often been studied in this structure. LTP is widely believed to be one of the main neural mechanisms by which memory is stored in the brain.
Hippocampus Anatomy
In terms of anatomy, the hippocampus is an elaboration of the edge of the cerebral cortex. The structures that line the edge of the cortex make up the so-called limbic system (Latin limbus = border).
These include the hippocampus, cingulate cortex, olfactory cortex, and amygdala. Paul MacLean once suggested, as part of his triune brain theory, that the limbic structures comprise the neural basis of emotion. Some neuroscientists no longer believe that the concept of a unified “limbic system” is valid, however.
Yet, the hippocampus is anatomically connected to parts of the brain that are involved with emotional behavior—the septum, the hypothalamic mammillary body, and the anterior nuclear complex in the thalamus—therefore its role as a limbic structure cannot be completely dismissed.
The hippocampus as a whole has the shape of a curved tube, which has been variously compared to a seahorse, a ram’s horn (Cornu Ammonis, hence the subdivisions CA1 through CA4), or a banana.
It can be distinguished as a zone where the cortex narrows into a single layer of densely packed pyramidal neurons 3 to 6 cells deep in rats, which curl into a tight U shape; one edge of the “U,” field CA4, is embedded into a backward-facing, strongly flexed, V-shaped cortex, the dentate gyrus. It consists of ventral and dorsal portions, both of which are of similar composition but are parts of different neural circuits.
This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. In the rat, the two hippocampi resemble a pair of bananas, joined at the stems by the hippocampal commissure that crosses the midline under the anterior corpus callosum.
In human or monkey brains, the portion of the hippocampus down at the bottom, near the base of the temporal lobe, is much broader than the part at the top. One of the consequences of this complex geometry is that cross-sections through the hippocampus can show a variety of shapes, depending on the angle and location of the cut.
Hippocampus Functions
Historically, the earliest widely held hypothesis was that the hippocampus is involved in olfaction. This idea was cast into doubt by a series of anatomical studies that did not find any direct projections to the hippocampus from the olfactory bulb.
However, later work did confirm that the olfactory bulb does project into the ventral part of the lateral entorhinal cortex, and field CA1 in the ventral hippocampus sends axons to the main olfactory bulb, the anterior olfactory nucleus, and to the primary olfactory cortex. There continues to be some interest in hippocampal olfactory responses, in particular the role of the hippocampus in memory for odors, but few specialists today believe that olfaction is its primary function.
Over the years, three main ideas of hippocampal function have dominated the literature: inhibition, memory, and space.
The behavioral inhibition theory (caricatured by O’Keefe and Nadel as “slam on the brakes!”) was very popular up to the 1960s. It derived much of its justification from two observations: first, that animals with hippocampal damage tend to be hyperactive; second, that animals with hippocampal damage often have difficulty learning to inhibit responses that they have previously been taught, especially if the response requires remaining quiet as in a passive avoidance test. Jeffrey Gray developed this line of thought into a full-fledged theory of the role of the hippocampus in anxiety. The inhibition theory is currently the least popular of the three.
The second major line of thought relates the hippocampus to memory. Although it had historical precursors, this idea derived its main impetus from a famous report by William Beecher Scoville and Brenda Milner describing the results of surgical destruction of the hippocampi (in an attempt to relieve epileptic seizures), in Henry Molaison, known until his death in 2008 as “Patient H.M.”
The unexpected outcome of the surgery was severe anterograde and partial retrograde amnesia; Molaison was unable to form new episodic memories after his surgery and could not remember any events that occurred just before his surgery, but he did retain memories of events that occurred many years earlier extending back into his childhood. This case attracted such widespread professional interest that Molaison became the most intensively studied subject in medical history.
In the ensuing years, other patients with similar levels of hippocampal damage and amnesia (caused by accident or disease) have also been studied, and thousands of experiments have studied the physiology of activity-driven changes in synaptic connections in the hippocampus. There is now almost universal agreement that the hippocampi play some sort of important role in memory; however, the precise nature of this role remains widely debated.
The third important theory of hippocampal function relates the hippocampus to space. The spatial theory was originally championed by O’Keefe and Nadel, who were influenced by E.C. Tolman’s theories about “cognitive maps” in humans and animals. O’Keefe and his student Dostrovsky in 1971 discovered neurons in the rat hippocampus that appeared to them to show activity related to the rat’s location within its environment.
Despite skepticism from other investigators, O’Keefe and his co-workers, especially Lynn Nadel, continued to investigate this question, in a line of work that eventually led to their very influential 1978 book The Hippocampus as a Cognitive Map. As with the memory theory, there is now almost universal agreement that spatial coding plays an important role in hippocampal function, but the details are widely debated.
Role in Memory
Psychologists and neuroscientists generally agree that the hippocampus plays an important role in the formation of new memories about experienced events (episodic or autobiographical memory). Part of this function is hippocampal involvement in the detection of novel events, places and stimuli. Some researchers regard the hippocampus as part of a larger medial temporal lobe memory system responsible for general declarative memory (memories that can be explicitly verbalized—these would include, for example, memory for facts in addition to episodic memory).
Due to bilateral symmetry the brain has a hippocampus in each cerebral hemisphere, so every normal brain has two of them. If damage to the hippocampus occurs in only one hemisphere, leaving the structure intact in the other hemisphere, the brain can retain near-normal memory functioning.
Severe damage to the hippocampi in both hemispheres results in profound difficulties in forming new memories (anterograde amnesia) and often also affects memories formed before the damage occurred (retrograde amnesia). Although the retrograde effect normally extends many years back before the brain damage, in some cases older memories remain. This retention of older memories leads to the idea that consolidation over time involves the transfer of memories out of the hippocampus to other parts of the brain.
Damage to the hippocampus does not affect some types of memory, such as the ability to learn new skills (playing a musical instrument or solving certain types of puzzles, for example). This fact suggests that such abilities depend on different types of memory (procedural memory) and different brain regions.
Furthermore, amnesic patients frequently show “implicit” memory for experiences even in the absence of conscious knowledge. For example, patients asked to guess which of two faces they have seen most recently may give the correct answer most of the time in spite of stating that they have never seen either of the faces before. Some researchers distinguish between conscious recollection, which depends on the hippocampus, and familiarity, which depends on portions of the medial temporal cortex.
Role in Spatial Memory and Navigation
Studies conducted on freely moving rats and mice have shown that many hippocampal neurons have “place fields”, that is, they fire bursts of action potentials when a rat passes through a particular part of the environment. Evidence for place cells in primates is limited, perhaps in part because it is difficult to record brain activity from freely moving monkeys.
Place-related hippocampal neural activity has been reported in monkeys moving around inside a room while seated in a restraint chair; on the other hand, Edmund Rolls and his colleagues instead described hippocampal cells that fire in relation to the place a monkey is looking at, rather than the place where its body is located.
In humans, cells with location-specific firing patterns have been reported in a study of patients with drug-resistant epilepsy who were undergoing an invasive procedure to localize the source of their seizures, with a view to surgical resection. The patients had diagnostic electrodes implanted in their hippocampus and then used a computer to move around in a virtual reality town.
Place responses in rats and mice have been studied in hundreds of experiments over four decades, yielding a large quantity of information. Place cell responses are shown by pyramidal cells in the hippocampus proper, and granule cells in the dentate gyrus. These constitute the great majority of neurons in the densely packed hippocampal layers. Inhibitory interneurons, which make up most of the remaining cell population, frequently show significant place-related variations in firing rate that are much weaker than those displayed by pyramidal or granule cells.
There is little if any spatial topography in the representation; in general, cells lying next to each other in the hippocampus have uncorrelated spatial firing patterns. Place cells are typically almost silent when a rat is moving around outside the place field but reach sustained rates as high as 40 Hertz when the rat is near the center. Neural activity sampled from 30 to 40 randomly chosen place cells carries enough information to allow a rat’s location to be reconstructed with high confidence.
The size of place fields varies in a gradient along the length of the hippocampus, with cells at the dorsal end showing the smallest fields, cells near the center showing larger fields, and cells at the ventral tip fields that cover the entire environment. In some cases, the firing rate of rat hippocampal cells depends not only on place but also on the direction a rat is moving, the destination toward which it is traveling, or other task-related variables.
The discovery of place cells in the 1970s led to a theory that the hippocampus might act as a cognitive map—a neural representation of the layout of the environment. Several lines of evidence support the hypothesis. It is a frequent observation that without a fully functional hippocampus, humans may not remember where they have been and how to get where they are going: Getting lost is one of the most common symptoms of amnesia.
Studies with animals have shown that an intact hippocampus is required for initial learning and long-term retention of some spatial memory tasks, in particular ones that require finding the way to a hidden goal. The “cognitive map hypothesis” has been further advanced by recent discoveries of head direction cells, grid cells, and border cells in several parts of the rodent brain that are strongly connected to the hippocampus.
Brain imaging shows that people have more active hippocampi when correctly navigating, as tested in a computer-simulated “virtual” navigation task. Also, there is evidence that the hippocampus plays a role in finding shortcuts and new routes between familiar places. For example, London’s taxi drivers must learn a large number of places and the most direct routes between them (they have to pass a strict test, The Knowledge, before being licensed to drive the famous black cabs).
A study at University College London by Maguire, et al.. (2000) showed that part of the hippocampus is larger in taxi drivers than in the general public, and that more experienced drivers have bigger hippocampi. Whether having a bigger hippocampus helps an individual to become a better cab driver, or if finding shortcuts for a living makes an individual’s hippocampus grow is yet to be elucidated.
However, in that study, Maguire et al. examined the correlation between size of the grey matter and length of time that had been spent as a taxi driver, and found a positive correlation between the length of time an individual had spent as a taxi driver and the volume of the right hippocampus. It was found that the total volume of the hippocampus remained constant, from the control group vs. taxi drivers. That is to say that the posterior portion of a taxi driver’s hippocampus is indeed increased, but at the expense of the anterior portion. There have been no known detrimental effects reported from this disparity in hippocampal proportions.
Physiology
The hippocampus shows two major “modes” of activity, each associated with a distinct pattern of neural population activity and waves of electrical activity as measured by an electroencephalogram (EEG). These modes are named after the EEG patterns associated with them: theta and large irregular activity (LIA). The main characteristics described below are for the rat, which is the animal most extensively studied.
The theta mode appears during states of active, alert behavior (especially locomotion), and also during REM (dreaming) sleep. In the theta mode, the EEG is dominated by large regular waves with a frequency range of 6 to 9 Hertz, and the main groups of hippocampal neurons (pyramidal cells and granule cells) show sparse population activity, which means that in any short time interval, the great majority of cells are silent, while the small remaining fraction fire at relatively high rates, up to 50 spikes in one second for the most active of them.
An active cell typically stays active for half a second to a few seconds. As the rat behaves, the active cells fall silent and new cells become active, but the overall percentage of active cells remains more or less constant. In many situations, cell activity is determined largely by the spatial location of the animal, but other behavioral variables also clearly influence it.
The LIA mode appears during slow-wave (non-dreaming) sleep, and also during states of waking immobility such as resting or eating. In the LIA mode, the EEG is dominated by sharp waves that are randomly timed large deflections of the EEG signal lasting for 25–50 milliseconds.
Sharp waves are frequently generated in sets, with sets containing up to 5 or more individual sharp waves and lasting up to 500 ms. The spiking activity of neurons within the hippocampus is highly correlated with sharp wave activity. Most neurons decrease their firing rate between sharp waves; however, during a sharp wave, there is a dramatic increase of firing rate in up to 10% of the hippocampal population
These two hippocampal activity modes can be seen in primates as well as rats, with the exception that it has been difficult to see robust theta rhythmicity in the primate hippocampus. There are, however, qualitatively similar sharp waves and similar state-dependent changes in neural population activity.
Hippocampus Theta Rhythm
Because of its densely packed neural layers, the hippocampus generates some of the largest EEG signals of any brain structure. In some situations the EEG is dominated by regular waves at 3 to 10 Hertz, often continuing for many seconds. These reflect subthreshold membrane potentials and strongly modulate the spiking of hippocampal neurons and synchronise across the hippocampus in a travelling wave pattern. This EEG pattern is known as a theta rhythm.
Theta rhythmicity is very obvious in rabbits and rodents and also clearly present in cats and dogs. Whether theta can be seen in primates is a vexing question. In rats (the animals that have been the most extensively studied), theta is seen mainly in two conditions: first, when an animal is walking or in some other way actively interacting with its surroundings; second, during REM sleep.
The function of theta has not yet been convincingly explained although numerous theories have been proposed. The most popular hypothesis has been to relate it to learning and memory. An example would be the phase with which theta rhythms, at the time of stimulation of a neuron, shape the effect of that stimulation upon its synapses. What is meant here is that theta rhythms may affect those aspects of learning and memory that are dependent upon synaptic plasticity.
It is well established that lesions of the medial septum—the central node of the theta system—cause severe disruptions of memory. However, the medial septum is more than just the controller of theta; it is also the main source of cholinergic projections to the hippocampus. It has not been established that septal lesions exert their effects specifically by eliminating the theta rhythm.
Further Reading:
Amaral, D; Lavenex P (2006). “Ch 3. Hippocampal Neuroanatomy”. In Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book . Oxford University Press. ISBN 978-0-19-510027-3.
Finger, S (2001). Origins of Neuroscience: A History of Explorations into Brain Function. Oxford University Press US.
Dori Derdikman and James J. Knierim, ed. (2014). Space,Time and Memory in the Hippocampal Formation. Springer
Howard Eichenbaum (2002). The Cognitive Neuroscience of Memory. Oxford University Press US.
Philippe Taupin (2007). The Hippocampus: Neurotransmission and Plasticity in the Nervous System. Nova Publishers