Significant neuronal degeneration is one consequence of Alzheimer’s disease, which is projected to impact approximately 6.7 million Americans in 2023. However, the mechanisms underlying neuron death remain inadequately comprehended.
According to a new Northwestern Medicine study, RNA interference may be a crucial factor in Alzheimer’s disease. Scientists have identified brief strands of toxic RNAs that cause brain cell death and DNA damage in aged and Alzheimer’s brains for the first time. The decline in short strands of protective RNAs that occur with age may facilitate the development of Alzheimer’s disease, according to the scientists.
“Nobody has ever connected the activities of RNAs to Alzheimer’s. We found that in aging brain cells, the balance between toxic and protective sRNAs shifts toward toxic ones,”
said corresponding study author Marcus Peter, the Tom D. Spies Professor of Cancer Metabolism at Northwestern University Feinberg School of Medicine.
Cell Protection Aging
The study also found that older individuals with a superior memory capacity (known as SuperAgers) have higher amounts of protective short RNA strands in their brain cells. SuperAgers are individuals aged 80 and older with a memory capacity of individuals 20 to 30 years younger.
Northwestern’s discovery may have implications beyond Alzheimer’s.
“Our data provide a new explanation for why, in almost all neurodegenerative diseases, affected individuals have decades of symptom-free life and then the disease starts to set in gradually as cells lose their protection with age,”
Peter said. The findings also point to a new way for treating Alzheimer’s and potentially other neurodegenerative diseases.
New Approach to Treatment
Alzheimer’s disease is distinguished by the progressive occurrence of amyloid-beta plaques, tau neurofibrillary tangles, scarring, and eventual brain cell death.
“The overwhelming investment in Alzheimer’s drug discovery has been focused on two mechanisms: reducing amyloid plaque load in the brain—which is the hallmark of Alzheimer’s diagnosis and 70 to 80% of the effort—and preventing tau phosphorylation or tangles. However, treatments aimed at reducing amyloid plaques have not yet resulted in an effective treatment that is well tolerated,”
Peter said.
The new findings support the idea that stabilizing or increasing the amount of protective short RNAs in the brain could be a completely new approach to preventing or delaying Alzheimer’s disease or neurodegeneration in general.
Peter stated that such drugs exist, but they must be tested and improved in animal models.
The next phase of Peter’s research will involve identifying the precise role that toxic sRNAs play in the cell death observed in Alzheimer’s disease in various animal and cellular models as well as in the brains of patients. Additionally, better compounds that would either selectively increase the level of protective sRNAs or block the action of the toxic ones will be screened for.
Toxic and Protective Short RNA
Every cell’s nucleus contains DNA, which stores all of our genetic information. To convert this gene information into the building blocks of life, DNA must be turned into RNA, which is then processed by cell machinery to generate proteins. The majority of biological processes rely on RNA.
In addition to these long coding RNAs, there exist many small RNAs (sRNAs) that do not encode proteins. They perform various important tasks in the cell. One type of sRNA inhibits long coding RNAs by a process known as RNA interference, which silences the proteins for which the long RNAs code.
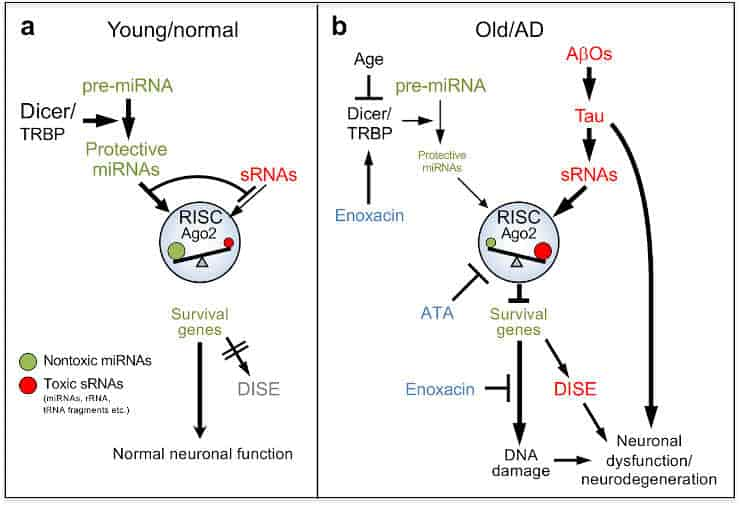
Peter and colleagues have now identified very short sequences found in some of these sRNAs that, when present, can kill cells by inhibiting the creation of proteins essential for cell survival, resulting in cell death. Their findings show that these hazardous sRNAs play a role in neuronal death, which contributes to Alzheimer’s disease.
The toxic sRNAs are normally inhibited by protective sRNAs. One type of sRNA is called microRNAs. While microRNAs play multiple important regulatory roles in cells, they are also the main species of protective sRNAs.
They act as guards, preventing harmful sRNAs from entering the cellular machinery responsible for RNA interference. However, the guardians’ numbers drop with age, allowing poisonous sRNAs to destroy the cells.
Scientists looked at the brains of Alzheimer’s disease mouse models, young and old mice, induced pluripotent stem cell-derived neurons from healthy people (young and old) and Alzheimer’s patients, the brains of seniors over 80 whose memory was the same as that of people 50 to 60 years old, and several human brain-derived neuron-like cell lines that had been treated with amyloid beta fragments, which are known to cause Alzheimer’s.
Abstract
Alzheimer’s disease (AD) is characterized by progressive neurodegeneration, but the specific events that cause cell death remain poorly understood. Death Induced by Survival gene Elimination (DISE) is a cell death mechanism mediated by short (s) RNAs acting through the RNA-induced silencing complex (RISC). DISE is thus a form of RNA interference, in which G-rich 6mer seed sequences in the sRNAs (position 2-7) target hundreds of C-rich 6mer seed matches in genes essential for cell survival, resulting in the activation of cell death pathways. Here, using Argonaute precipitation and RNAseq (Ago-RP-Seq), we analyze RISC-bound sRNAs to quantify 6mer seed toxicity in several model systems. In mouse AD models and aging brain, in induced pluripotent stem cell-derived neurons from AD patients, and in cells exposed to Aβ42 oligomers, RISC-bound sRNAs show a shift to more toxic 6mer seeds compared to controls. In contrast, in brains of “SuperAgers”, humans over age 80 who have superior memory performance, RISC-bound sRNAs are shifted to more nontoxic 6mer seeds. Cells depleted of nontoxic sRNAs are sensitized to Aβ42-induced cell death, and reintroducing nontoxic RNAs is protective. Altogether, the correlation between DISE and Aβ42 toxicity suggests that increasing the levels of nontoxic miRNAs in the brain or blocking the activity of toxic RISC-bound sRNAs could ameliorate neurodegeneration.
Reference:
- Paudel, B., Jeong, SY., Martinez, C.P. et al. Death Induced by Survival gene Elimination (DISE) correlates with neurotoxicity in Alzheimer’s disease and aging. Nat Commun 15, 264 (2024). Doi: 10.1038/s41467-023-44465-8
