In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another cell (neural or otherwise). Santiago Ramón y Cajal proposed that neurons are not continuous throughout the body, yet still communicate with each other, an idea known as the neuron doctrine.
The word “synapse” – from the Greek synapsis (συνάπσις), meaning “conjunction”, in turn from συνάπτεὶν (συν (“together”) and ἅπτειν (“to fasten”)) – was introduced in 1897 by English physiologist Michael Foster at the suggestion of English classical scholar Arthur Woollgar Verrall.
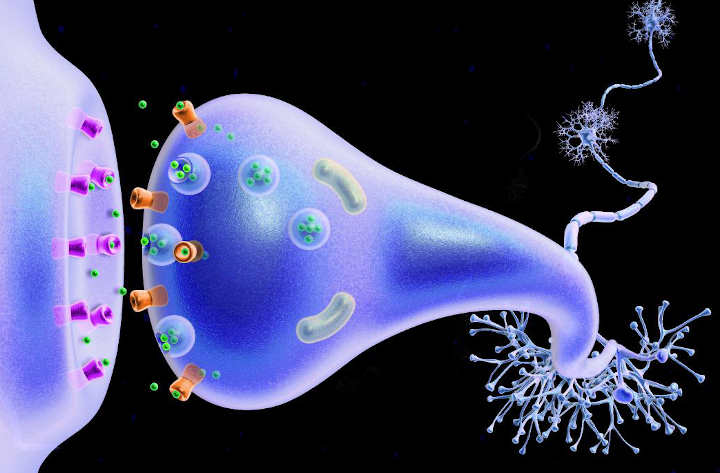
Synapses are essential to neuronal function: neurons are cells that are specialized to pass signals to individual target cells, and synapses are the means by which they do so. At a synapse, the plasma membrane of the signal-passing neuron (the presynaptic neuron) comes into close apposition with the membrane of the target (postsynaptic) cell.
Both the presynaptic and postsynaptic sites contain extensive arrays of molecular machinery that link the two membranes together and carry out the signaling process. In many synapses, the presynaptic part is located on an axon, but some postsynaptic sites are located on a dendrite or soma.
Astrocytes also exchange information with the synaptic neurons, responding to synaptic activity and, in turn, regulating neurotransmission.
Different Types of Synapses
In a chemical synapse, electrical activity in the presynaptic neuron is converted (via the activation of voltage-gated calcium channels) into the release of a chemical called a neurotransmitter that binds to receptors located in the plasma membrane of the postsynaptic cell.
The neurotransmitter may initiate an electrical response or a secondary messenger pathway that may either excite or inhibit the postsynaptic neuron.
Chemical synapses can be classified according to the neurotransmitter released:
- Glutamatergic (often excitatory)
- GABAergic (often inhibitory)
- Cholinergic (e.g. vertebrate neuromuscular junction)
- Adrenergic (releasing norepinephrine)
Because of the complexity of receptor signal transduction, chemical synapses can have complex effects on the postsynaptic cell.
In an electrical synapse, the presynaptic and postsynaptic cell membranes are connected by special channels called gap junctions that are capable of passing electric current, causing voltage changes in the presynaptic cell to induce voltage changes in the postsynaptic cell. The main advantage of an electrical synapse is the rapid transfer of signals from one cell to the next.
Synaptic communication is distinct from ephaptic coupling, in which communication between neurons occurs via indirect electric fields.
Role in Memory
It is widely accepted that the synapse plays a role in the formation of memory. As neurotransmitters activate receptors across the synaptic cleft, the connection between the two neurons is strengthened when both neurons are active at the same time, as a result of the receptor’s signalling mechanisms.
The strength of two connected neural pathways is thought to result in the storage of information, resulting in memory. This process of synaptic strengthening is known as long-term potentiation.
By altering the release of neurotransmitters, plasticity of synapses can be controlled in the presynaptic cell. The postsynaptic cell can be regulated by altering the function and number of its receptors.
Changes in postsynaptic signaling are most commonly associated with N-methyl-d-aspartic acid receptor (NMDAR)-dependent long-term potentiation (LTP) and long-term depression (LTD), which are the most analyzed forms of plasticity at excitatory synapses.
Synaptic Polarization
The function of neurons depends upon cellular polarization. The distinctive structure of nerve cells allows action potentials to travel directionally (from dendrites to axons), and for these signals to then be received and carried on by post-synaptic neurons or received by effector cells.
Nerve cells have long been used as models for cellular polarization, and of particular interest are the mechanisms underlying the polarized localization of synaptic molecules. PIP2 signalling regulated by IMPase plays an integral role in synaptic polarity.
Phosphoinositides (PIP, PIP2, and PIP3) are molecules that have been shown to affect neuronal polarity.
A gene (ttx-7) was identified in Caenorhabditis elegans that encodes myo-inositol monophosphatase (IMPase), an enzyme that produces inositol by dephosphorylating inositol phosphate. Organisms with mutant ttx-7 genes demonstrated behavioral and localization defects, which were rescued by expression of IMPase.
This led to the conclusion that IMPase is required for the correct localization of synaptic protein components. The egl-8 gene encodes a homolog of phospholipase Cβ (PLCβ), an enzyme that cleaves PIP2. When ttx-7 mutants also had a mutant egl-8 gene, the defects caused by the faulty ttx-7 gene were largely reversed. These results suggest that PIP2 signaling establishes polarized localization of synaptic components in living neurons.