Electrical stimulation of the spinal cord is feasible, well-tolerated, and has therapeutic potential for treating depression, according to a pilot clinical trial led by University of Cincinnati researchers at the Lindner Center of HOPE.The research of principal investigator Francisco Romo-Nava, MD, Ph.D., focuses on how the brain-body connection is involved in psychiatric disorders.
“We think that the connection between the brain and the body is essential for psychiatric disorders. Many of the symptoms of mood disorders or eating disorders or anxiety disorders have to do with what one could interpret as dysregulation in this brain-body interaction,”
said Romo-Nava, associate professor in the Department of Psychiatry and Behavioral Neurosciences at UC.
Brain-body Circuit Overload
Neuronal pathways in the spinal cord provide information from the body to brain regions involved in the emotional experience known as mood. When the brain is operating properly, it uses this information to constantly make modifications to help control a person’s mood.
While major depressive disorder can have many different causes, one contributor could be this pathway being overloaded with information.
For example, chronic stress could lead to a hyperactive brain-body circuit that eventually burns the system out and prevents it from adjusting itself in an effective and optimal way,
Romo-Nava said.
Decreasing the Noise
The researchers investigated various methods for modulating this relationship between the brain and the body and devised a novel strategy using noninvasive spinal cord stimulation. Romo-Nava received a patent for the stimulation method utilized in 2020 after collaborating with UC’s Office of Innovation.
The spinal cord stimulation is designed to decrease the flow of information in the brain-body circuit so that the brain is better able to readjust and regulate itself.
“Spinal cord stimulation is thought to help the brain modulate itself as it should by decreasing the noise or decreasing the hyperactive signaling that may be in place during a depressive syndrome,”
Romo-Nava said.
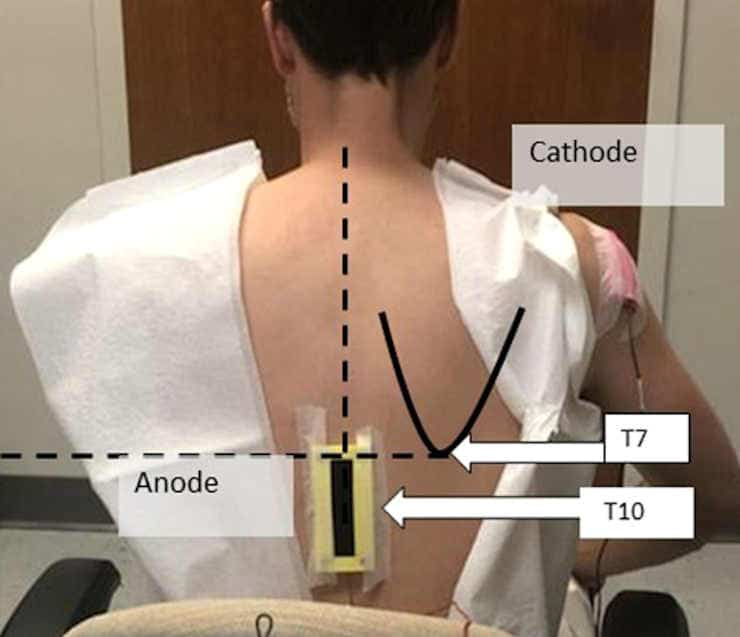
The investigational device that was used is no larger than a shoe box, with the active electrode placed on the patient’s back and the return electrode placed on their right shoulder.
Feasibility and Safety

Romo-Nava established the pilot study to evaluate the acceptability and feasibility of spinal cord stimulation for patients with major depressive disorder.
The trial included 20 patients, with half assigned to get the active version of spinal cord stimulation and the other half receiving an alternative version of current that was not predicted to have much of an effect.
Patients went to the Lindner Center of HOPE for three 20-minute sessions a week for eight weeks, for a total of 24 spinal stimulation sessions.
According to Romo-Nava, the primary emphasis of the trial, like with other pilot studies, was the feasibility and safety of the intervention, as well as how well patients tolerated the stimulation. The trial was structured so that the dose of stimulation may be reduced if necessary, but Romo-Nava said all patients tolerated the original given level well.
“We used a current that is so small that it’s about ten times smaller than the one known to induce tissue damage, so that’s also pretty encouraging because there’s a lot to explore in terms of what is the optimal dose and session frequency,”
he said.
Spinal Gray Matter
The treatment had only mild side effects, such as skin redness at the stimulation site and transient non-painful itching or burning sensations that persisted only during the treatment sessions. According to Romo-Nava, skin redness usually lasted no more than 20 minutes following a session.
A virtual reconstruction of how the current from the device moves through the body showed the current reaches spinal gray matter in the spinal cord but does not reach the brain itself.
“That supports our hypothesis that it is the modulation of these pathways of information that then may induce an effect on the mood-relevant areas in the brain,” he said. “So it is not the current that reaches the brain; it is the change in the signal that then has an effect. This study is not sufficient to prove all of these components of the hypothesis, but we think it’s a great start.”
Patients who received active stimulation demonstrated a greater decrease in the severity of their depression symptoms when compared to the control group, but Romo-Nava noted that the study’s small sample size was a drawback. To be confirmed, these findings will need to be replicated in much bigger trials.
“We need to be cautious when we interpret these results because of the pilot nature and the small sample size of the study. While the primary outcome was positive and it shows therapeutic potential, we should acknowledge all the limitations of the study,”
he said.
Next Steps
Data showed participants’ resting blood pressure did not change over the course of the eight weeks, but their diastolic blood pressure (the bottom number of a blood pressure reading) decreased for a short time after each session in a cumulative way during the study.
“That may mean that we may be actually inducing a form of plastic effect on the brain-body interaction circuit that is also involved in autonomic functions like blood pressure and heart rate,” Romo-Nava said. “This is very preliminary, but it is also another signal that is in the right direction.”
Moving forward, Romo-Nava said the research team is seeking additional funding to put together an expanded trial and develop a portable version of the spinal cord stimulation device. If further research confirms that the stimulation is safe and effective for treating psychiatric disorders, more research will be required to determine the optimal dose, frequency, and conditions under which it can be used.
Abstract
Converging theoretical frameworks suggest a role and a therapeutic potential for spinal interoceptive pathways in major depressive disorder (MDD). Here, we aimed to evaluate the antidepressant effects and tolerability of transcutaneous spinal direct current stimulation (tsDCS) in MDD. This was a double-blind, randomized, sham-controlled, parallel group, pilot clinical trial in unmedicated adults with moderate MDD. Twenty participants were randomly allocated (1:1 ratio) to receive “active” 2.5 mA or “sham” anodal tsDCS sessions with a thoracic (anode; T10)/right shoulder (cathode) electrode montage 3 times/week for 8 weeks. Change in depression severity (MADRS) scores (prespecified primary outcome) and secondary clinical outcomes were analyzed with ANOVA models. An E-Field model was generated using the active tsDCS parameters. Compared to sham (n = 9), the active tsDCS group (n = 10) showed a greater baseline to endpoint decrease in MADRS score with a large effect size (−14.6 ± 2.5 vs. −21.7 ± 2.3, p = 0.040, d = 0.86). Additionally, compared to sham, active tsDCS induced a greater decrease in MADRS “reported sadness” item (−1.8 ± 0.4 vs. −3.2 ± 0.4, p = 0.012), and a greater cumulative decrease in pre/post tsDCS session diastolic blood pressure change from baseline to endpoint (group difference: 7.9 ± 3.7 mmHg, p = 0.039). Statistical trends in the same direction were observed for MADRS “pessimistic thoughts” item and week-8 CGI-I scores. No group differences were observed in adverse events (AEs) and no serious AEs occurred. The current flow simulation showed electric field at strength within the neuromodulation range (max. ~0.45 V/m) reaching the thoracic spinal gray matter. The results from this pilot study suggest that tsDCS is feasible, well-tolerated, and shows therapeutic potential in MDD. This work also provides the initial framework for the cautious exploration of non-invasive spinal cord neuromodulation in the context of mental health research and therapeutics. The underlying mechanisms warrant further investigation. Clinicaltrials.gov registration: NCT03433339
Reference:
- Romo-Nava, F., Awosika, O.O., Basu, I. et al. Effect of non-invasive spinal cord stimulation in unmedicated adults with major depressive disorder: a pilot randomized controlled trial and induced current flow pattern. Mol Psychiatry (2023). Doi: 10.1038/s41380-023-02349-9