Scientists at the Karolinska Institutet in Sweden have elucidated the molecular logic that underlies the assembly of spinal circuits controlling the velocity of locomotion in adult zebrafish.
The adaptability of motor activities in terms of timing, speed, and strength is essential for rapid adaptation to the ever-changing world around us. The most obvious example of this is during locomotion, a behaviour that necessitates full-body coordination and is characterized by abrupt changes in speed and strength.
In this study, we used single-cell RNA sequencing in adult zebrafish to link the molecular diversity of motoneurons and interneurons with their modular circuit organization that is responsible for changes in locomotor speed. We show that each neuronal population comprises three specific subtypes defined by key molecular features and correspond to neurons underlying locomotion at slow, intermediate and fast speeds,”
said corresponding author Abdel El Manira, Professor at the Department of Neuroscience at Karolinska Institutet.
Motoneuron Diversity Underpinning
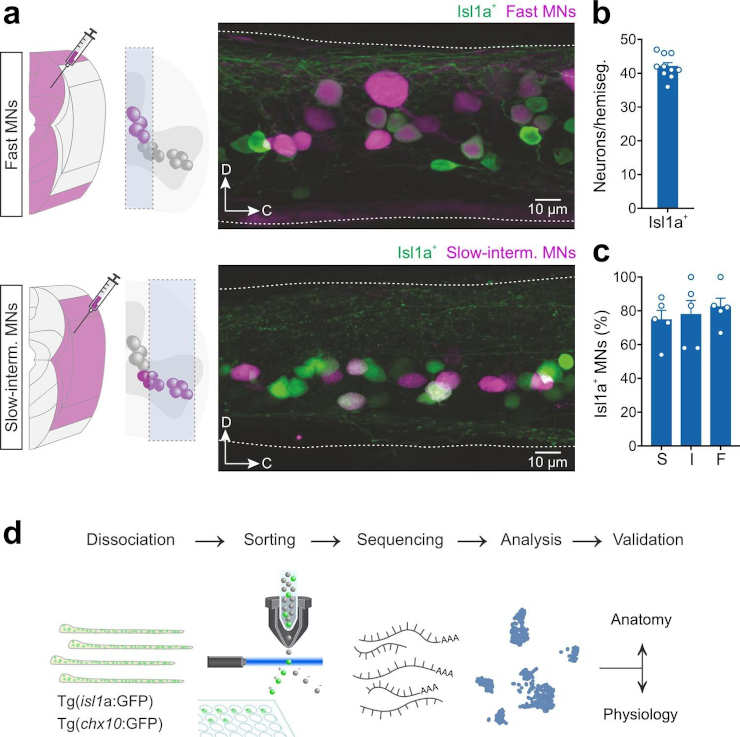
The researchers’ analysis also reveals molecular signatures that define each of the three circuit speed modules. The study uncovers the molecular underpinnings for neuronal diversity and how they relate to the function of locomotor circuits in adult zebrafish.
In general, the study adds a lot to our knowledge of the molecular and circuitry mechanisms that control locomotor flexibility. It also helps us map the circuits that control motor actions by showing how the different kinds of molecules in motoneurons and interneurons affect how they work, connect, and behave.
“While the categorization of muscle units into slow, intermediate, and fast types is universal to all vertebrates, the molecular underpinnings of motoneuron diversity and their premotor circuits have remained unclear. Our study fills this critical gap in our knowledge and represents a true advance in the field,”
said Irene Pallucchi, co-author of the article.
Modular Circuit Organization
The conceptual advance provided by the study is of broad interest to researchers in the motor control field and brain circuit organization in general.
“We used single-cell RNA sequencing, electrophysiology, anatomical, and behavioral analysis in adult zebrafish, which provide both experimental and genetic accessibility,”
explained Maria Bertuzzi, research engineer in Abdel El Manira group and co-author of the article.
This helped them figure out the molecular and functional differences between motoneuron and interneuron subtypes, as well as how their modular circuits are set up to control how fast they move.
The study’s molecularly specified three-speed circuit module organization functions as gearshift mechanisms for speed change and can also allow for quick changes in direction. However, it is unknown how the brainstem drives the varied speed circuit modules.
“Our future studies aim to test the hypothesis that the commands from the brain are channeled to the spinal locomotor network through distinct command streams, each of which preferentially controls one of the speed circuit modules,”
said Abdel El Manira.
By establishing such an arrangement, task and situation-dependent locomotor maneuverability and flexibility would be significantly enhanced.
Abstract
The flexibility of motor actions is ingrained in the diversity of neurons and how they are organized into functional circuit modules, yet our knowledge of the molecular underpinning of motor circuit modularity remains limited. Here we use adult zebrafish to link the molecular diversity of motoneurons (MNs) and the rhythm-generating V2a interneurons (INs) with the modular circuit organization that is responsible for changes in locomotor speed. We show that the molecular diversity of MNs and V2a INs reflects their functional segregation into slow, intermediate or fast subtypes. Furthermore, we reveal shared molecular signatures between V2a INs and MNs of the three speed circuit modules. Overall, by characterizing how the molecular diversity of MNs and V2a INs relates to their function, connectivity and behavior, our study provides important insights not only into the molecular mechanisms for neuronal and circuit diversity for locomotor flexibility but also for charting circuits for motor actions in general.
Reference:
- Pallucchi, I., Bertuzzi, M., Madrid, D. et al. Molecular blueprints for spinal circuit modules controlling locomotor speed in zebrafish. Nat Neurosci (2023). doi: 10.1038/s41593-023-01479-1
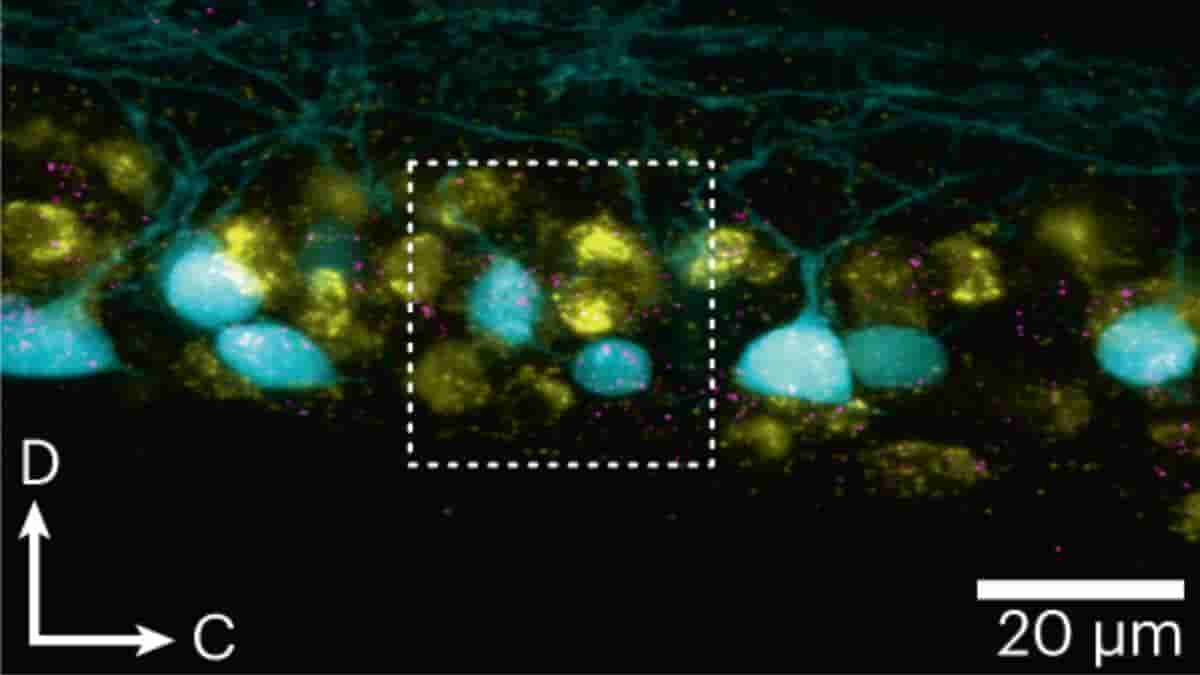
Top Image: Lateral view of a spinal cord segment showing RNAscope in situ hybridization of grin1b and pvalb6 in slow–intermediate motor neurons. Credit: Nat Neurosci (2023). doi: 10.1038/s41593-023-01479-1
Last Updated on November 13, 2023