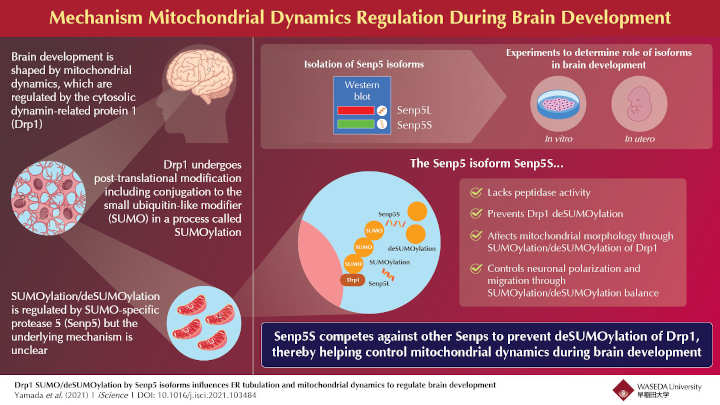
Mitochondrial dynamics are mainly regulated by dynamin-related protein 1 (Drp1). The molecular mechanisms that affect the functioning of Drp1 influence brain development. But little has been known on how exactly this happens.
A recent study1 describes a newly discovered protein in the brain that helps regulate Drp1 and thus, brain function. The finding offers new perspective on the pathology of many developmental and neurological disorders, which could help design effective treatments and therapies for diseases like Alzheimer’s and Parkinson’s in the future.
Drp1 undergoes a modification called ‘SUMOylation,’ which studies have shown to play a key role in many neurological disorders like Alzheimer’s and Parkinson’s. The protein we discovered targets this SUMOylation process, which makes it a very attractive option for potential therapies for SUMOylation-related diseases,
said Professor Shin-ichi Sakakibara, of Waseda University, who led the research group.
Small Ubiquitin-like Modifier
Understanding how the brain develops is crucial to formulating treatments and management protocols for a variety of developmental disorders, as well as degenerative neurological diseases. Right from the embryonic stage, brain development is facilitated by the differentiation of neurons.
These fundamental processes are regulated by the dynamics of mitochondria. These dynamics encompass mitochondrial fission, by which a mitochondrion divides, and mitochondrial fusion, which helps mitochondria elongate.
After Drp1 proteins are “translated,” or made, they undergo a modification by a protein called small ubiquitin-like modifier (SUMO). SUMOylated Drp1s are tagged by the body for degradation2.
Tagged Drp1s are later “untagged” to control the number of these proteins being degraded. This process is called deSUMOylation. Previous research has shown that deSUMOylation is catalyzed by a variant of an enzyme called SUMO-specific protease 5 (Senp5). This variant is called Senp5L and it helps break the bond between Drp1 and SUMO.
Mitochondrial Morphology
The research team discovered another variant of Senp5 that they named Senp5S. They then performed in vitro experiments using cell lines and in utero experiments using mouse embryos to study the effects of Senp5S and SUMOylation on mitochondrial dynamics and neuronal differentiation.
The researchers found that unlike Senp5L, Senp5S had no “bond-breaking” (peptidase) activity. Instead, it competed with Senp5L at the reaction site and prevented deSUMOylation of Drp1 proteins, thereby indirectly regulating mitochondrial dynamics.
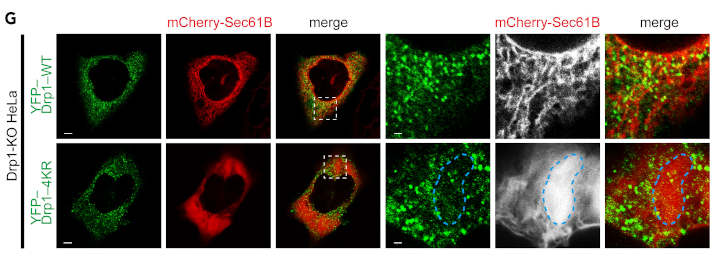
The research team further found that the SUMOylation/deSUMOylation balance affected mitochondrial morphology as well as the tabulation of another crucial cellular structure that helps in protein production and assembly in the cell — the endoplasmic reticulum.
Balanced Senp5L/Senp5S expression are essential for brain development. Our findings suggest a novel and vital role for post-translational SUMOylation in the tightly controlled process of neuronal differentiation and brain development. It also helps clarify the physiological significance of SUMOylation in the brain,
said Sakakibara.
The study does have some limitations to be aware of. First, tag-conjugated SUMO3 to was used to show Senp5 L/S-dependent control of Drp1 SUMOylation status. Given that Drp1-4KR is not able to be SUMOylated with SUMO3 as well as SUMO1 and that Senp5L/S inhibits/promotes global SUMO1-ylation, not only SUMO3 but also SUMO1 is possibly involved in Senp5 L/S-dependent control of Drp1 SUMOylation and mitochondrial dynamics.
Secondly, the scientists demonstrated crucial roles of Senp5L/S in the regulation of mitochondrial dynamics and corticogenesis with a genetical approach including isoform-specific gain-of-function. Nevertheless, sh/siRNA for isoform-specific loss of function and antibodies that separately recognize each isoform would advance our understanding on physiological significance of Senp5 isoforms
- Seiya Yamada, Ayaka Sato, Naotada Ishihara, Hiroki Akiyama, Shin-ichi Sakakibari. Drp1 SUMO/deSUMOylation by Senp5 isoforms influences ER tubulation and mitochondrial dynamics to regulate brain development, iScience, Volume 24, Issue 12, 103484, December 17, 2021 ↩︎
- Pichler A., Fatouros C., Lee H., Eisenhardt N. SUMO conjugation – a mechanistic view. Biomol. Concepts. 2017; 8: 13-36 ↩︎
Last Updated on October 27, 2022