From absorbing new languages to mastering musical instruments, young children are wired to learn in ways that adults are not (Johnson and Newport, 1989). This ability coincides with periods of intense brain plasticity during which neurons can easily remodel their connections (Hubel and Wiesel, 1970).
Many children are also scandalously good sleepers, typically getting several more hours of sleep per night than their parents (Jenni and Carskadon, 2007). As sleep deprivation has negative effects on learning and memory, learning like a child likely requires sleeping like one (Diekelmann and Born, 2010).
Yet, how the ability to sleep for longer is synchronized with windows of high brain plasticity is not fully understood.
Genetic Regulatory Mechanism
Sleep is deeply conserved through evolution, and examining how it develops in ‘simple organisms’ should provide fundamental insights relevant to humans. For instance, like human teenagers, one-day old Drosophila melanogaster flies sleep twice as much as mature adults, and disrupting the sleep of young flies has lasting effects on learning and behavior (Seugnet et al., 2011; Kayser et al., 2014).
Now, Matthew Kayser from the University of Pennsylvania and co-workers – including Leela Chakravarti Dilley as first author – report new regulatory mechanisms that promote sleep in young flies1.
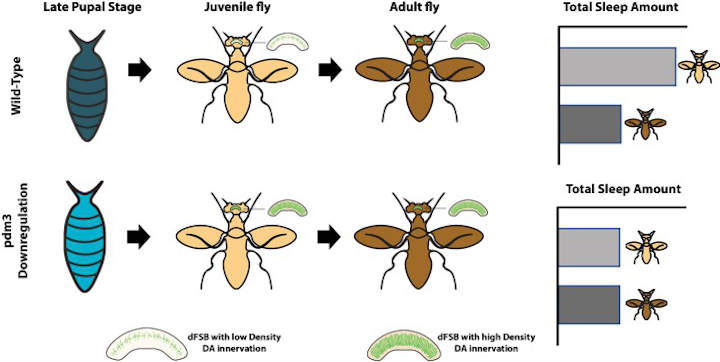
The team started by searching for genes which, when knocked down, would reduce the difference in sleep duration between younger and older adult flies. A gene called pdm3 fit the bill by reducing sleep in juveniles.
This gene codes for a transcription factor that belongs to a family known to regulate normal brain development. Chakravarti Dilley et al. further determined that pdm3 helps to establish correct sleep patterns for one-day-old flies during the pupal stage, when the relatively simple brain of a larva develops into the complex brain of the adult insect – a period of radical change that puts human puberty to shame.
Transcription Factor Knockout
Previous work on pdm3 mutants revealed aberrations in the way dopaminergic neurons reach and connect with neurons in the central complex, a region of the brain that is known to regulate sleep. There, the dopaminergic neurons encourage wakefulness by inhibiting cells called dFSB neurons, which promote sleep (Pimentel et al., 2016).
In flies, the density of connections between dopaminergic and dFSB neurons normally increases over the first few days of adult life.
Chakravarti Dilley et al. therefore explored whether pdm3 might regulate how dopaminergic neurons innervate the central complex. This revealed that when pdm3 was knocked down, one-day-old flies already showed levels of innervation that rivaled those seen in mature adults (Figure 1).
Given that pdm3 encodes a transcription factor, the team then searched for genes that regulate sleep and whose expression was altered by pdm3 being knocked down. These experiments suggested that pdm3 suppresses the expression of msp300, a gene from a family involved in synapse maturation.
And indeed, knocking down both pdm3 and msp300 resulted in flies that developed normally in terms of sleep patterns and dopaminergic innervation of the central complex.
Perturbing neural development, especially during windows of high plasticity, can have a long-lasting impact on the ability for the brain to work properly (Marín, 2016). A lack of sleep could lead to such perturbations, as evidenced by the fact that disrupting sleep in early childhood or adolescence has long-term effects on behavior (Taveras et al., 2017; Roberts et al., 2009).
Understanding how sleep is synchronized with periods of intense development may help to develop better therapeutic interventions that lessen long-term brain damage.
- L Chakravarti Dilley, M Szuperak, NN Gong, CE Williams, RL Saldana, DS Garbe, MH Syed, R Jain, MS Kayser (2020). Identification of a molecular basis for the juvenile sleep state. eLife 9:e52676. ↩︎
Last Updated on October 17, 2022