Molecularly targeted therapy, or targeted therapy, is one of the major modalities of medical treatment for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells (e.g. with traditional chemotherapy).
Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy (and thus distinguished from chemotherapy, that is, cytotoxic therapy). However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
Another form of molecularly targeted therapy involves the use of nanoengineered enzymes to bind to a tumor cell such that the body’s natural cell degradation process can digest the cell, effectively eliminating it from the body.
There are targeted therapies for lung cancer, colorectal cancer, head and neck cancer, breast cancer, multiple myeloma, lymphoma, prostate cancer, pancreatic cancer, melanoma and other cancers.
Benefits Of Molecularly Targeted Therapy
Targeted cancer therapies are expected to be more effective than older forms of treatments and less harmful to normal cells. Biomarkers are usually required to aid the selection of patients who will likely respond to a given targeted therapy.
Many molecularly targeted therapies are examples of immunotherapy (using immune mechanisms for therapeutic goals) developed by the field of cancer immunology. Thus, as immunomodulators, they are one type of biological response modifiers.
The most successful targeted therapies are chemical entities that target or preferentially target a protein or enzyme that carries a mutation or other genetic alteration that is specific to cancer cells and not found in normal host tissue.
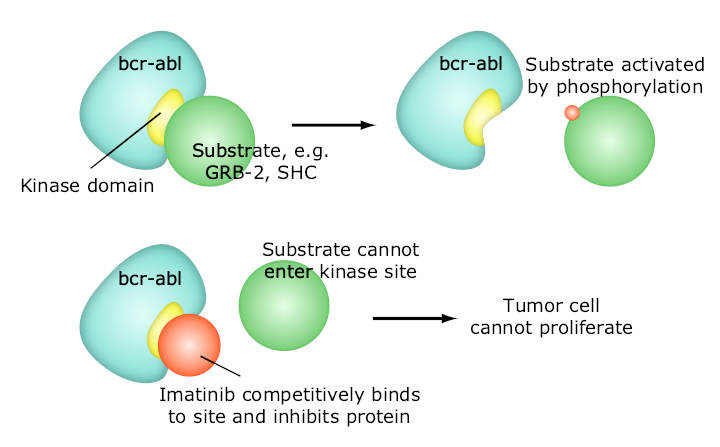
One of the most successful molecular targeted therapeutic is Gleevec, which is a kinase inhibitor with exceptional affinity for the oncofusion protein BCR-Abl which is a strong driver of tumorigenesis in chronic myelogenous leukemia. Although employed in other indications, Gleevec is most effective targeting BCR-Abl.
Other examples of molecular targeted therapeutics targeting mutated oncogenes, include PLX27892 which targets mutant B-raf in melanoma.
Co-targeted therapy involves the use of one or more therapeutics aimed at multiple targets, for example PI3K and MEK, in an attempt to generate a synergistic response1 and prevent the development of drug resistance.
The definitive experiments that showed that targeted therapy would reverse the malignant phenotype of tumor cells involved treating Her2/neu transformed cells with monoclonal antibodies in vitro and in vivo by Mark Greene’s laboratory and reported from 19852.
Types Of Molecularly Targeted Therapy
The main categories of targeted therapy are currently small molecules and monoclonal antibodies.
Small Molecules
- Imatinib (Gleevec, also known as STI–571) is approved for chronic myelogenous leukemia, gastrointestinal stromal tumor and some other types of cancer. Early clinical trials indicate that imatinib may be effective in treatment of dermatofibrosarcoma protuberans.
- Gefitinib (Iressa, also known as ZD1839), targets the epidermal growth factor receptor (EGFR) tyrosine kinase and is approved in the U.S. for non-small cell lung cancer.
- Erlotinib (marketed as Tarceva). Erlotinib inhibits epidermal growth factor receptor, and works through a similar mechanism as gefitinib. Erlotinib has been shown to increase survival in metastatic non-small cell lung cancer when used as second line therapy. Because of this finding, erlotinib has replaced gefitinib in this setting.
- Sorafenib (Nexavar) a kinase inhibitor drug approved for the treatment of primary kidney cancer (advanced renal cell carcinoma), advanced primary liver cancer (hepatocellular carcinoma), FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.
- Sunitinib (Sutent)
- Dasatinib (Sprycel)
- Lapatinib (Tykerb), a dual tyrosine kinase inhibitor which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways. It is used in combination therapy for HER2-positive breast cancer.
- Nilotinib (Tasigna)
- Bortezomib (Velcade) is an apoptosis-inducing proteasome inhibitor drug that causes cancer cells to undergo cell death by interfering with proteins. It is approved in the U.S. to treat multiple myeloma that has not responded to other treatments.
- The selective estrogen receptor modulator tamoxifen has been described as the foundation of targeted therapy.
- Janus kinase inhibitors, e.g. FDA approved tofacitinib
- ALK inhibitors, e.g. Crizotinib
- Bcl-2 inhibitors (e.g. FDA approved venetoclax, obatoclax in clinical trials, navitoclax, and gossypol.
- PARP inhibitors (e.g. FDA approved olaparib, rucaparib, niraparib and talazoparib)
- PI3K inhibitors (e.g. perifosine in a phase III trial)
- Apatinib is a selective VEGF Receptor 2 inhibitor which has shown encouraging anti-tumor activity in a broad range of malignancies in clinical trials. Apatinib is currently in clinical development for metastatic gastric carcinoma, metastatic breast cancer and advanced hepatocellular carcinoma.
- Zoptarelin doxorubicin (AN-152), doxorubicin linked to [D-Lys(6)]- LHRH, Phase II results for ovarian cancer
- Braf inhibitors (vemurafenib, dabrafenib, LGX818) used to treat metastatic melanoma that harbors BRAF V600E mutation
- MEK inhibitors (trametinib, MEK162) are used in experiments, often in combination with BRAF inhibitors to treat melanoma
- CDK inhibitors, e.g. PD-0332991, LEE011 in clinical trials
- Hsp90 inhibitors, some in clinical trials
- Hedgehog pathway inhibitors (e.g. FDA approved vismodegib and sonidegib).
- salinomycin has demonstrated potency in killing cancer stem cells in both laboratory-created and naturally occurring breast tumors in mice.
Small Molecules: Serine/threonine Kinase Inhibitors
- Temsirolimus (Torisel)
- Everolimus (Afinitor)
- Vemurafenib (Zelboraf)
- Trametinib (Mekinist)
- Dabrafenib (Tafinlar)
Monoclonal Antibodies
Several are in development and a few have been licensed by the FDA and the European Commission. Examples of licensed monoclonal antibodies include:
- Pembrolizumab (Keytruda) binds to PD-1 proteins found on T cells. Pembrolizumab blocks PD-1 and help the immune system kill cancer cells. It is used to treat melanoma, Hodgkin’s lymphoma, non-small cell lung carcinoma and several other types of cancer.
- Rituximab targets CD20 found on B cells. It is used in non Hodgkin lymphoma
- Trastuzumab targets the Her2/neu (also known as ErbB2) receptor expressed in some types of breast cancer
- Alemtuzumab
- Cetuximab targets the epidermal growth factor receptor (EGFR). It is approved for use in the treatment of metastatic colorectal cancer and squamous cell carcinoma of the head and neck.
- Panitumumab also targets the EGFR. It is approved for the use in the treatment of metastatic colorectal cancer.
- Bevacizumab targets circulating VEGF ligand. It is approved for use in the treatment of colon cancer, breast cancer, non-small cell lung cancer, and is investigational in the treatment of sarcoma. Its use for the treatment of brain tumors has been recommended.
- Ipilimumab (Yervoy)
- Heavey S, O’Byrne KJ, Gately K (April 2014). Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treatment Reviews. 40 (3): 445–56. ↩︎
- Perantoni AO, Rice JM, Reed CD, Watatani M, Wenk ML (September 1987). Activated neu oncogene sequences in primary tumors of the peripheral nervous system induced in rats by transplacental exposure to ethylnitrosourea. Proceedings of the National Academy of Sciences of the United States of America. 84 (17): 6317–21 ↩︎
Last Updated on October 26, 2022