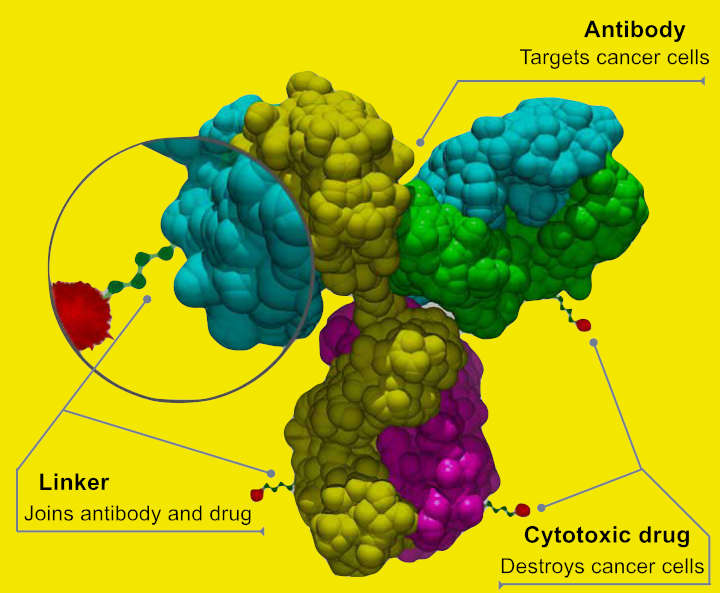
Antibody-drug conjugates (ADCs) are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.
Antibody-drug conjugates are complex molecules composed of an antibody linked to a biologically anticancer payload or drug. ADCs combine the targeting capabilities of monoclonal antibodies with the cancer-killing ability of cytotoxic drugs. They can be designed to discriminate between healthy and diseased tissue.
How Antibody-drug Conjugates Work
An anticancer drug is coupled to an antibody that specifically targets a certain tumor antigen (e.g. a protein that, ideally, is only to be found in or on tumor cells). Antibodies attach themselves to the antigens on the surface of cancerous cells.
The biochemical reaction between the antibody and the target protein (antigen) triggers a signal in the tumor cell, which then absorbs or internalizes the antibody together with the linked cytotoxin. After the ADC is internalized, the cytotoxin kills the cancer. This targeting limits side effects and gives a wider therapeutic window than other chemotherapeutic agents.
A stable link between the antibody and cytotoxic (anti-cancer) agent is a crucial aspect of an ADC. A stable ADC linker ensures that less of the cytotoxic payload falls off before reaching a tumor cell, improving safety, and limiting dosages.
ADC Linkers
Linkers are based on chemical motifs including disulfides, hydrazones or peptides (cleavable), or thioethers (noncleavable). Cleavable and noncleavable linkers were proved to be safe in preclinical and clinical trials.
Brentuximab vedotin includes an enzyme-sensitive cleavable linker that delivers the antimicrotubule agent monomethyl auristatin E or MMAE, a synthetic antineoplastic agent, to human-specific CD30-positive malignant cells.
MMAE inhibits cell division by blocking the polymerization of tubulin. Because of its high toxicity MMAE cannot be used as a single-agent chemotherapeutic drug.
However, MMAE linked to an anti-CD30 monoclonal antibody (cAC10, a cell membrane protein of the tumor necrosis factor or TNF receptor) was stable in extracellular fluid. It is cleavable by cathepsin and safe for therapy.
Trastuzumab emtansine is a combination of the microtubule-formation inhibitor mertansine (DM-1) and antibody trastuzumab that employs a stable, non-cleavable linker.
The availability of better and more stable linkers has changed the function of the chemical bond. The type of linker, cleavable or noncleavable, lends specific properties to the cytotoxic drug.
For example, a non-cleavable linker keeps the drug within the cell. As a result, the entire antibody, linker and cytotoxic (anti-cancer) agent enter the targeted cancer cell where the antibody is degraded into an amino acid. The resulting complex – amino acid, linker and cytotoxic agent – is considered to be the active drug.
In contrast, cleavable linkers are detached by enzymes in the cancer cell. The cytotoxic payload can then escape from the targeted cell and, in a process called bystander killing1, attack neighboring cells.
History Of Antibody-drug Conjugates
Drugs that would target tumor cells and ignore others was conceived in 1900 by German Nobel laureate Paul Ehrlich.
In 2001 Pfizer/Wyeth’s drug Gemtuzumab ozogamicin (trade name: Mylotarg) was approved based on a study with a surrogate endpoint, through the accelerated approval process. In June 2010, after evidence accumulated showing no evidence of benefit and significant toxicity, the U.S. Food and Drug Administration (FDA) forced the company to withdraw it. It was reintroduced into the US market in 2017.
Brentuximab vedotin (trade name: Adcetris, marketed by Seattle Genetics and Millennium/Takeda) was approved for relapsed HL and relapsed sALCL by the FDA on August 19, 2011 and received conditional marketing authorization from the European Medicines Agency in October 2012.
Trastuzumab emtansine (ado-trastuzumab emtansine or T-DM1, trade name: Kadcyla, marketed by Genentech and Roche) was approved in February 2013 for the treatment of people with HER2-positive metastatic breast cancer (mBC) who had received prior treatment with trastuzumab and a taxane chemotherapy.
The European Commission approved Inotuzumab ozogamicin2 as a monotherapy for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL) on June 30, 2017 under the trade name Besponsa (Pfizer/Wyeth), followed on August 17, 2017 by the FDA.
The first immunology antibody-drug conjugate (iADC), ABBV-3373, showed an improvement in disease activity in a Phase 2a clinical trial of patients with rheumatoid arthritis and a study with the second iADC, ABBV-154 to evaluate adverse events and change in disease activity in participants treated with subcutaneous injection of ABBV-154 is ongoing.
- Kovtun YV, Goldmacher VS (October 2007). Cell killing by antibody-drug conjugates. Cancer Letters. 255 (2): 232–40 ↩︎
- Ricart AD (October 2011). Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin. Clinical Cancer Research. 17 (20): 6417–27 ↩︎
Last Updated on October 26, 2022