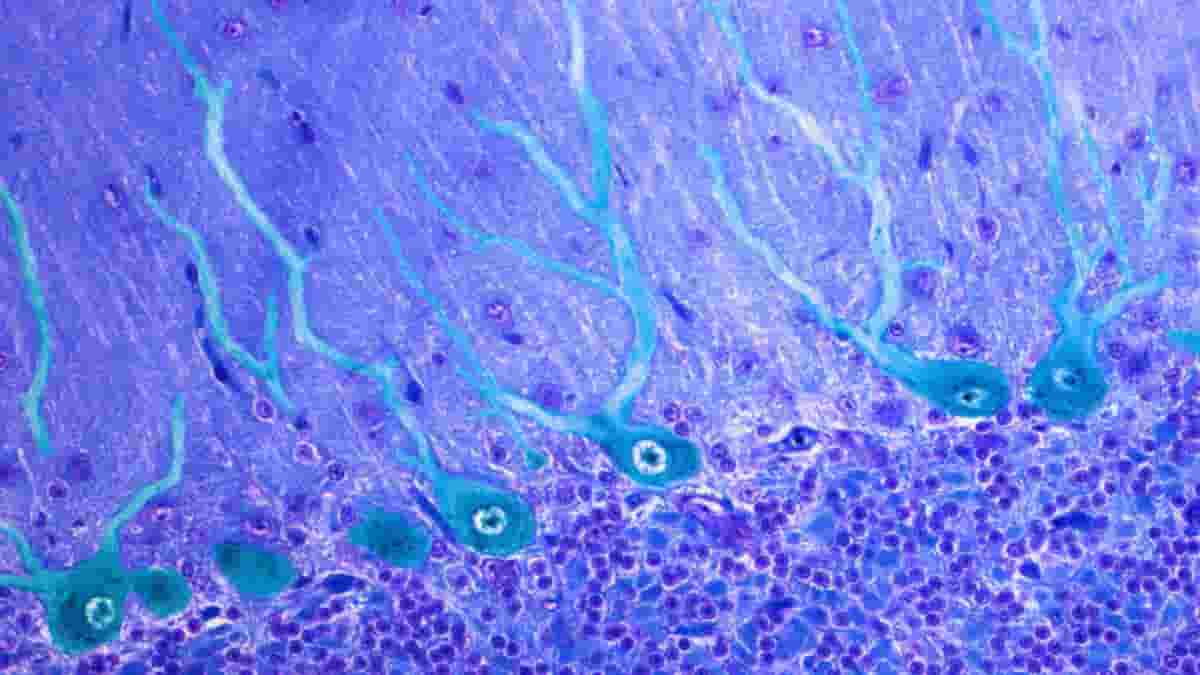
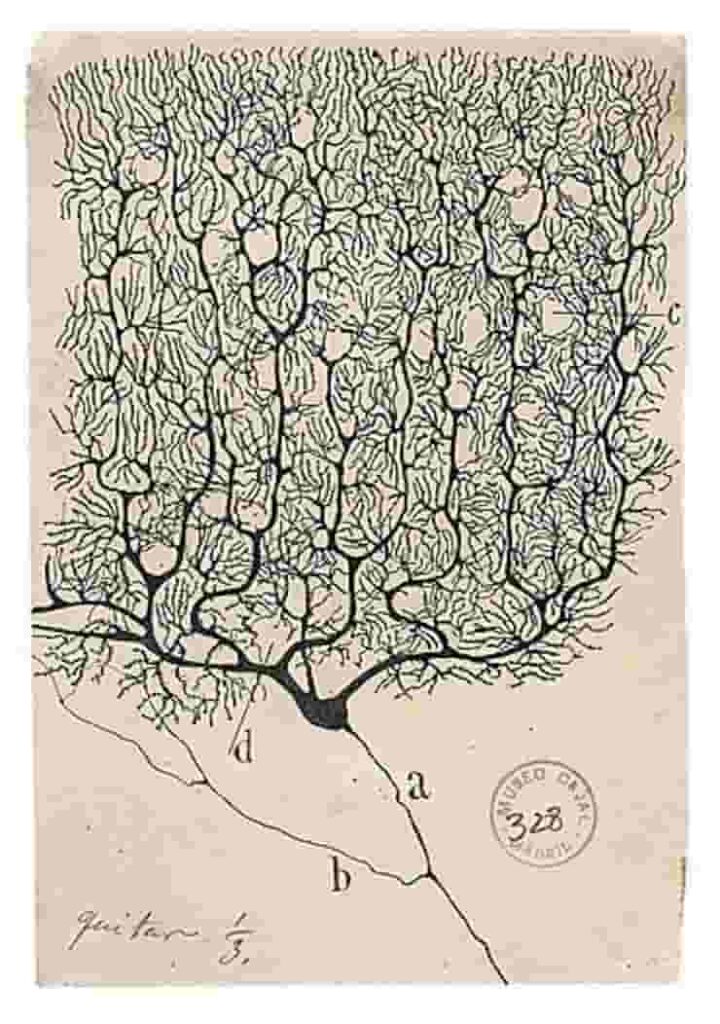
Santiago Ramón y Cajal, a Spanish scientist, was awarded the Nobel Prize in 1906 for his pioneering studies of the microscopic structures of the brain. His famous drawings of Purkinje cells in the cerebellum depict a forest of neuron structures, with multiple large branches sprouting from the cell body and splitting into lovely, leaf-like patterns.
Although these early depictions showed multiple dendrites extending from the cell body, the current prevailing belief among neuroscientists is that Purkinje cells have only one primary dendrite that connects with a single climbing fiber from the brain stem.
However, new research shows that Cajal’s drawings were correct all along — nearly all Purkinje cells in the human cerebellum have multiple primary dendrites.
Further research in mice revealed that approximately 50% of their Purkinje cells have this more complex structure, and 25% of these cells receive input from multiple climbing fibers that connect with different primary dendrite branches. Experiments using live mice to record cell activity revealed that the primary branches can be activated independently, responding to different environmental stimuli.
“The more you work with a certain prototype of a cell in your mind, the more you accept it. These drawings by Cajal have been around since the 1900s, so we definitely had enough time to pay attention, but only now with this quantitative analysis do we see that it’s almost universal that human cells have multiple full dendrites each, and we can see that it makes a qualitative difference too,”
said senior author Christian Hansel, Ph.D., Professor of Neurobiology at the University of Chicago.
Cerebellum Role
The cerebellum is located at the cranium base, just above where the spinal cord connects. Since French physician Jean Pierre Flourens first described the cerebellum’s function in 1824, scientists assumed that its sole function was coordinating movement and muscular activity.
However, technological advances have revealed that the cerebellum also plays an important role in processing input about the body’s internal and external environments, including sensations of proprioception and balance.
Cerebellar Purkinje cells function similarly to large antennae, receiving thousands of inputs from the rest of the body and transmitting a wide range of contextual information. These signals are then combined with a prediction-error signal to detect a mismatch between the context and the brain’s expectation.
Climbing Fiber Dogma
This error signal is delivered by nerve fibers that ascend from the brain stem and connect with their intended Purkinje dendrite structures. These nerves are appropriately referred to as “climbing fibers.”
The conventional wisdom holds that each Purkinje cell has one primary dendrite that branches from the cell body and connects with one climbing fiber, forming a single computational unit.
Belief in this one-to-one relationship between climbing fibers and Purkinje cells, a central dogma in the field found in every neuroscience textbook, stems primarily from studies on rodents, which have the single dendrite configuration.
Many previous studies of these structures have focused on small numbers of cells, so Silas Busch, a Ph.D. student in Hansel’s lab and the paper’s first author, began by looking at thousands of cells from both human and mouse tissue.
Multiple Primary Dendrites
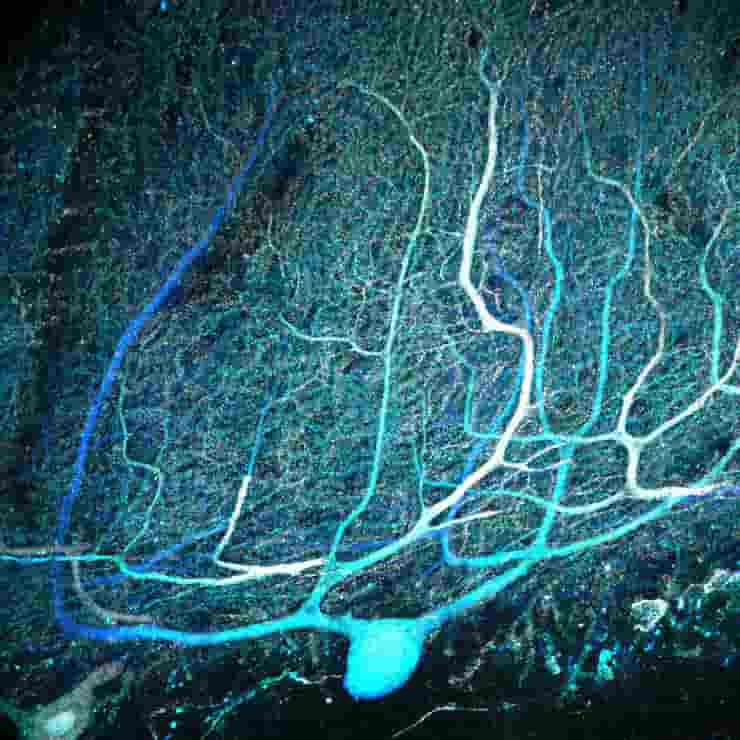
Busch used immunohistochemistry, a targeted antibody-based staining technique, to selectively label Purkinje cells in thin slices of cerebellum. He then classified the structure of all the cells he could see.
Busch discovered that more than 95% of human Purkinje cells had multiple primary dendrites, whereas in mice, the figure was much closer to half.
“You get a sense for how much this was a prevailing idea in the field because anatomically, they are referred to as the primary dendrite of a cell. So, even the description of the structure of these cells is based on that mouse prototype that has one dendrite you can call a primary dendrite,”
Busch said.
This remarkable species difference in one of the most evolutionarily conserved brain areas shared by mammals and even other vertebrates prompted Busch and Hansel to wonder if there was a functional benefit to having multiple primary dendrites rather than just one. Their first suspect was the climbing fiber, which had an advantageous one-to-one relationship and was intimately entangled with the primary dendrite.
Rewriting the Textbooks
Busch used sections of mouse cerebellum containing still living cells and dyed the cells to see their branches before stimulating climbing fiber inputs. He discovered that 25% of cells with multiple primary dendrites received multiple climbing fibers, rewriting the textbook assumption that each Purkinje cell receives only one climbing fiber input, whereas cells with a single primary dendrite did not.
Encouraged by the discovery that a sizable portion — albeit a minority — of Purkinje cells with multiple primary dendrites also received input from multiple climbing fibers, Busch conducted a series of experiments in living mice to see if it resulted in functional differences in the live mouse.
He first injected a fluorescent calcium indicator dye into the cerebellum and implanted a small glass window to observe the flow of calcium into the Purkinje cell dendrites later. He could measure calcium flow by restraining the mouse’s head under a microscope while it ran on a treadmill, which indicated when a climbing fiber was providing a strong input to the cell.
Wiggling Whiskers on Sleeping Mice
High-resolution images of cells with one primary dendrite revealed that the activity signal was uniform across its structure; in cells with multiple primary dendrites, he could detect activity on each side occurring at different times, implying that one dendrite could be activated by its climbing fiber while the other dendrite in the same cell was not.
Busch then wanted to see if he could isolate individual climbing fiber activity using a more precise stimulus: the mouse’s whiskers. Busch had to sedate the mice for this experiment.
“I don’t know if you’ve ever tried to stimulate individual whiskers in an awake mouse, but it’s really hard,”
he said.
Busch threaded individual whiskers into a small glass tube and wiggled them back and forth while the mice slept. He could also see activity in Purkinje cell dendritic branches, implying that individual climbing fibers signalled input from individual whiskers to individual dendrites.
Finally, Busch tested awake mice with stimuli such as light flashes, sounds, and air puffs on the whisker pad to simulate a more realistic scenario.
Again, he noticed differences between Purkinje cells. In some cases, one branch may prefer one stimulus over another, so it may be particularly sensitive to light but not sound. The other branch may then respond preferentially to sound but not light.
“This happened in a minority of cells since there are fewer with multiple branches in mice, and not all of them get multiple climbing fibers, but still, the presence of this effect was very interesting. It confirmed this idea that the two climbing fiber inputs will have different functional purposes that represent different information,”
Busch said.
Beyond Motor Coordination and Adaptation
This new evidence challenges conventional wisdom about a brain area thought to be anatomically well-defined and has functional implications. Purkinje cells aggregate and process information from climbing fibers from the brain stem.
Multiple inputs connected at multiple points on the cells provide more computational power, allowing brain circuits to adapt and respond to changes in the environment or the body that necessitate different movements, and this non-canonical connectivity is closely linked to the structure of Purkinje cell dendrites.
There is also evidence that these cerebellar connections are involved in disease. Hansel, for example, collaborated with U Chicago neurologist Christopher Gomez, MD, Ph.D. in 2013 on a study that found Purkinje-climbing fiber connections are weaker in mouse models of cerebellar ataxia, a movement disorder.
Busch, Hansel, and Gomez, on the other hand, have published research with former UChicago graduate student Dana Simmons showing that these connections are stronger in genetic duplication and overexpression models of autism.
Other researchers have found stronger links in certain types of tremors as well. Understanding these cells’ essential biological structures should provide more insight into these conditions.
“People who study other parts of the brain like the neocortex or the hippocampus always have more or less an idea of what that brain structure is doing. Those of us who study the cerebellum always had this idea that it’s motor coordination and adaptation, but it was also clear that it was something beyond that. Now it will be easier to grasp as the connectivity becomes clearer,”
Hansel concluded.
References:
- Silas E. Busch et al. Climbing fiber multi-innervation of mouse Purkinje dendrites with arborization common to human. Science (2023). DOI: 10.1126/science.adi1024
- Du, X., Wang, J., Zhu, H., Rinaldo, L., Lamar, K. M., Palmenberg, A. C., Hansel, C., & Gomez, C. M. (2013). Second cistron in CACNA1A gene encodes a transcription factor mediating cerebellar development and SCA6. Cell, 154(1), 118–133
- Simmons, D. H., Busch, S. E., Titley, H. K., Grasselli, G., Shih, J., Du, X., Wei, C., Gomez, C. M., Piochon, C., & Hansel, C. (2021). Sensory Over-responsivity and Aberrant Plasticity in Cerebellar Cortex in a Mouse Model of Syndromic Autism. Biological psychiatry global open science, 2(4), 450–459
Last Updated on September 18, 2023