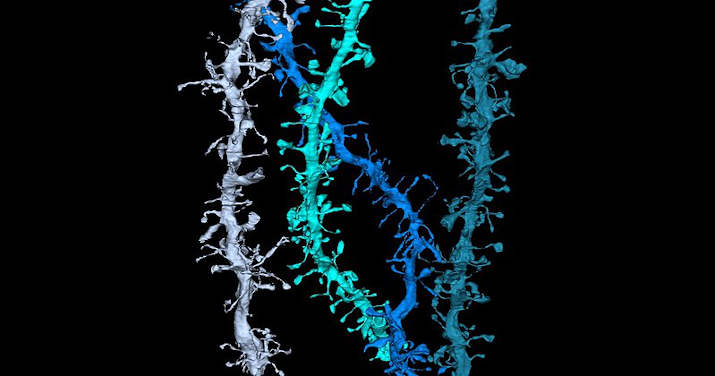
Dendrites are the branched projections of a neuron that act to propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which they project.
Electrical stimulation is transmitted onto them by upstream neurons (usually their axons) via synapses which are located at various points throughout the dendritic tree.
Dendrites play a critical role in
- integrating these synaptic inputs
- determining the extent to which action potentials are produced by the neuron.
Dendritic arborization, also known as dendritic branching, is a multi-step biological process by which neurons form new dendritic trees and branches to create new synapses.
The morphology, such as branch density and grouping patterns are highly correlated to the function of the neuron1. Malformation of dendrites is also tightly correlated to impaired nervous system function.
Dendritic Spines
Certain classes of dendrites contain small projections referred to as dendritic spines that increase receptive properties of dendrites to isolate signal specificity. Increased neural activity and the establishment of long-term potentiation at dendritic spines change the size, shape, and conduction.
This ability for dendritic growth is thought to play a role in learning and memory formation. There can be as many as 15,000 spines per cell, each of which serves as a postsynaptic process for individual presynaptic axons.
Dendritic branching can be extensive and in some cases is sufficient to receive as many as 100,000 inputs to a single neuron.
Dendrites and Axons
Dendrites are one of two types of protoplasmic protrusions that extrude from the cell body of a neuron, the other type being an axon. Axons can be distinguished from dendrites by several features including shape, length, and function.
Dendrites often taper off in shape and are shorter, while axons tend to maintain a constant radius and be relatively long.
Typically, axons transmit electrochemical signals and dendrites receive the electrochemical signals, although some types of neurons in certain species lack axons and simply transmit signals via their dendrites.
Dendrites provide an enlarged surface area to receive signals from the terminal buttons of other axons, and the axon also commonly divides at its far end into many branches (telodendria) each of which ends in a nerve terminal, allowing for a chemical signal to pass simultaneously to many target cells.
Typically, when an electrochemical signal stimulates a neuron, it occurs at a dendrite and causes changes in the electrical potential across the neuron’s plasma membrane. This change in the membrane potential will passively spread across the dendrite but becomes weaker with distance without an action potential.
An action potential propagates the electrical activity along the membrane of the neuron’s dendrites to the cell body and then afferently down the length of the axon to the axon terminal, where it triggers the release of neurotransmitters into the synaptic cleft. However, synapses involving dendrites can also be axodendritic, involving an axon signaling to a dendrite, or dendrodendritic, involving signaling between dendrites.
Development Of Dendrites
During development, several factors can influence differentiation. These include modulation of sensory input, environmental pollutants, body temperature, and drug use.
For example, rats raised in dark environments were found to have a reduced number of spines in pyramidal cells located in the primary visual cortex and a marked change in distribution of dendrite branching in layer 4 stellate cells. Experiments done in vitro and in vivo have shown that the presence of afferents and input activity per se can modulate the patterns in which dendrites differentiate.
Little is known about the process by which they orient themselves in vivo and are compelled to create the intricate branching pattern unique to each specific neuronal class. One theory on the mechanism of dendritic arbor development is the Synaptotropic Hypothesis.
The synaptotropic hypothesis proposes that input from a presynaptic to a postsynaptic cell (and maturation of excitatory synaptic inputs) eventually can change the course of synapse formation at dendritic and axonal arbors.
This synapse formation is required for the development of neuronal structure in the functioning brain. A balance between metabolic costs of dendritic elaboration and the need to cover receptive field presumably determine the size and shape of dendrites.
A complex array of extracellular and intracellular cues modulates development including transcription factors, receptor-ligand interactions, various signaling pathways, local translational machinery, cytoskeletal elements, Golgi outposts and endosomes. These contribute to the organization of the dendrites on individual cell bodies and the placement of these dendrites in the neuronal circuitry.
For example, it was shown that β-actin zipcode binding protein 1 (ZBP1) contributes to proper dendritic branching. Other important transcription factors involved in the morphology of dendrites include CUT, Abrupt, Collier, Spineless, ACJ6/drifter, CREST, NEUROD1, CREB, NEUROG2 etc. Secreted proteins and cell surface receptors includes neurotrophins and tyrosine kinase receptors, BMP7, Wnt/dishevelled, EPHB 1-3, Semaphorin/plexin-neuropilin, slit-robo, netrin-frazzled, reelin. Rac, CDC42 and RhoA serve as cytoskeletal regulators and the motor protein includes KIF5, dynein, LIS1.
Important secretory and endocytic pathways controlling the dendritic development include DAR3 /SAR1, DAR2/Sec23, DAR6/Rab1 etc. All these molecules interplay with each other in controlling dendritic morphogenesis including the acquisition of type specific dendritic arborization, the regulation of dendrite size and the organization of dendrites emanating from different neurons.
Plasticity
Dendrites themselves appear to be capable of plastic changes during the adult life of animals, including invertebrates. Neuronal dendrites have various compartments known as functional units that are able to compute incoming stimuli.
These functional units are involved in processing input and are composed of the subdomains of dendrites such as spines, branches, or groupings of branches. Therefore, plasticity that leads to changes in the dendrite structure will affect communication and processing in the cell.
During development, dendrite morphology is shaped by intrinsic programs within the cell’s genome and extrinsic factors such as signals from other cells. But in adult life, extrinsic signals become more influential and cause more significant changes in dendrite structure compared to intrinsic signals during development.
In females, the dendritic structure can change as a result of physiological conditions induced by hormones during periods such as pregnancy, lactation, and following the estrous cycle. This is particularly visible in pyramidal cells of the CA1 region of the hippocampus, where the density of dendrites can vary up to 30%.
-
Pinel, John P.J. (2011)
Biopsychology (8th ed.)
Boston: Allyn & Bacon. ISBN 978-0-205-83256-9 ↩︎