A team of scientists recently discovered a microbial compound that damages dopamine-producing neurons. Further research revealed that this causes Parkinson-like symptoms.
The finding provides fresh insight into the possibility of environmental factors, including metabolites from the human microbiome, acting as Parkinson’s disease triggers.
This research was led by Thomas Böttcher from the Institute of Biological Chemistry and the Center for Microbiology and Environmental Systems Science (CeMESS) at the University of Vienna and Marcel Leist from the University of Konstanz, in collaboration with the Albert Einstein College of Medicine.
Environmental Factors
The underlying causes of Parkinson’s, a serious neurodegenerative disease, remain obscure. While genetic mutations are known to be a cause of Parkinson’s, 90% of cases occur sporadically, with no clear genetic origin.
Environmental factors, including industrial chemicals and pesticides, are being investigated for potential links to neurodegeneration because scientists believe that these substances may have an impact.
Recent research emphasizes the importance of the gut-brain axis and suggests that the human microbiome may have an impact on neurodegenerative illnesses. The term “human microbiome” refers to all bacteria that can inhabit the human body.
The intestinal microbiome of Parkinson’s patients differs from that of healthy people. Some microbial metabolites specifically attack dopamine-producing neurons, which are critically affected in Parkinson’s disease.
Streptomyces Venezuelae
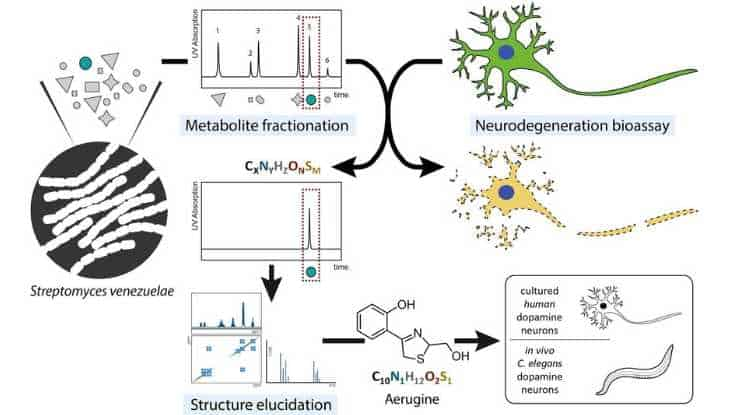
The current study concentrated on a metabolite known as aerugine generated by the bacteria Streptomyces venezuelae. The researchers isolated and identified this molecule before exposing it to dopamine-producing neurons in humans.
The findings were unambiguous: the metabolite had a deleterious effect that caused neuronal loss akin to that of Parkinson’s disease.
To further validate their findings, the researchers examined the effect of this bacterial metabolite on nematodes, which subsequently showed movement difficulties and specific neuronal patterns similar to those of human Parkinson’s patients.
Tangible Link
This groundbreaking study, which combines the domains of microbial biochemistry and molecular neuroscience, was coauthored by Leist from the University of Konstanz and Böttcher from the University of Vienna.
“Our research establishes a tangible link between a specific bacterial metabolite and symptoms similar to Parkinson’s. It is a further step in understanding how our environment, down to the microbes around us, influence the onset or progression of such diseases could,”
said Leist.
This discovery creates new research opportunities in addition to providing a fresh viewpoint on the factors that cause Parkinson’s disease.
- Could other microbial substances influence neurodegenerative diseases?
- How do these substances interact with our neurons?
- And most importantly, can this knowledge lead to new treatments or preventative measures?
“Although the study is just a beginning, it is a promising step towards unraveling the molecular causes of Parkinson’s and other neurodegenerative diseases,”
said Thomas Böttcher.
Abstract
The causes of nigrostriatal cell death in idiopathic Parkinson’s disease are unknown, but exposure to toxic chemicals may play some role. We followed up here on suggestions that bacterial secondary metabolites might be selectively cytotoxic to dopaminergic neurons. Extracts from Streptomyces venezuelae were found to kill human dopaminergic neurons (LUHMES cells). Utilizing this model system as a bioassay, we identified a bacterial metabolite known as aerugine (C10H11NO2S; 2-[4-(hydroxymethyl)-4,5-dihydro-1,3-thiazol-2-yl]phenol) and confirmed this finding by chemical re-synthesis. This 2-hydroxyphenyl-thiazoline compound was previously shown to be a product of a wide-spread biosynthetic cluster also found in the human microbiome and in several pathogens. Aerugine triggered half-maximal dopaminergic neurotoxicity at 3–4 µM. It was less toxic for other neurons (10–20 µM), and non-toxic (at <100 µM) for common human cell lines. Neurotoxicity was completely prevented by several iron chelators, by distinct anti-oxidants and by a caspase inhibitor. In the Caenorhabditis elegans model organism, general survival was not affected by aerugine concentrations up to 100 µM. When transgenic worms, expressing green fluorescent protein only in their dopamine neurons, were exposed to aerugine, specific neurodegeneration was observed. The toxicant also exerted functional dopaminergic toxicity in nematodes as determined by the “basal slowing response” assay. Thus, our research has unveiled a bacterial metabolite with a remarkably selective toxicity toward human dopaminergic neurons in vitro and for the dopaminergic nervous system of Caenorhabditis elegans in vivo. These findings suggest that microbe-derived environmental chemicals should be further investigated for their role in the pathogenesis of Parkinson’s disease.
Reference:
- Ückert, Anna-Katharina et al. Identification of the bacterial metabolite aerugine as potential trigger of human dopaminergic neurodegeneration. Environment international, vol. 180 108229. 23 Sep. 2023, doi:10.1016/j.envint.2023.108229
Image credit: Environment International (2023). DOI: 10.1016/j.envint.2023.108229
Last Updated on November 11, 2023