While prior research has suggested that Parkinson’s disease begins in the gut and travels to the brain, the exact mechanism has remained a mystery. A new pre-clinical study led by Duke University researchers adds to the body of evidence supporting the gut-brain relationship.
The researchers describe a process in which a protein found in the gut called alpha-synuclein travels through the nervous system and reaches vulnerable nerves in the brain.
“It’s when alpha-synuclein proteins become corrupted that this transport system becomes an issue,” “If they are corrupted in the gut and are then able to spread to the brain, they could form clumps known as Lewy bodies, which are the hallmark of Parkinson’s disease and other forms of dementia,”
said senior author Rodger Liddle, M.D., professor in the Department of Medicine at Duke University School of Medicine.
Enteroendocrine Cells
Parkinson’s disease is a long-term degenerative condition that inhibits voluntary movement. It is estimated that up to ten million people globally are infected with the disease.
There is growing evidence that the gut plays a role in developing Parkinson’s. One clue is that gastrointestinal symptoms such as constipation often happen before motor skills decline.
Liddle and colleagues concentrated on enteroendocrine cells, which are specialized cells that lining the gut. These cells respond to their surroundings and detect toxicants such as herbicides and pesticides in the intestine; they also contain alpha-synuclein.
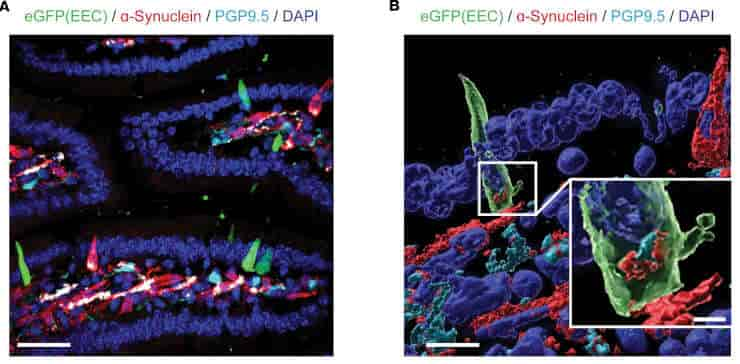
Credit: JCI Insight (2023). DOI: 10.1172/jci.insight.172192
Vagus Nerve Route
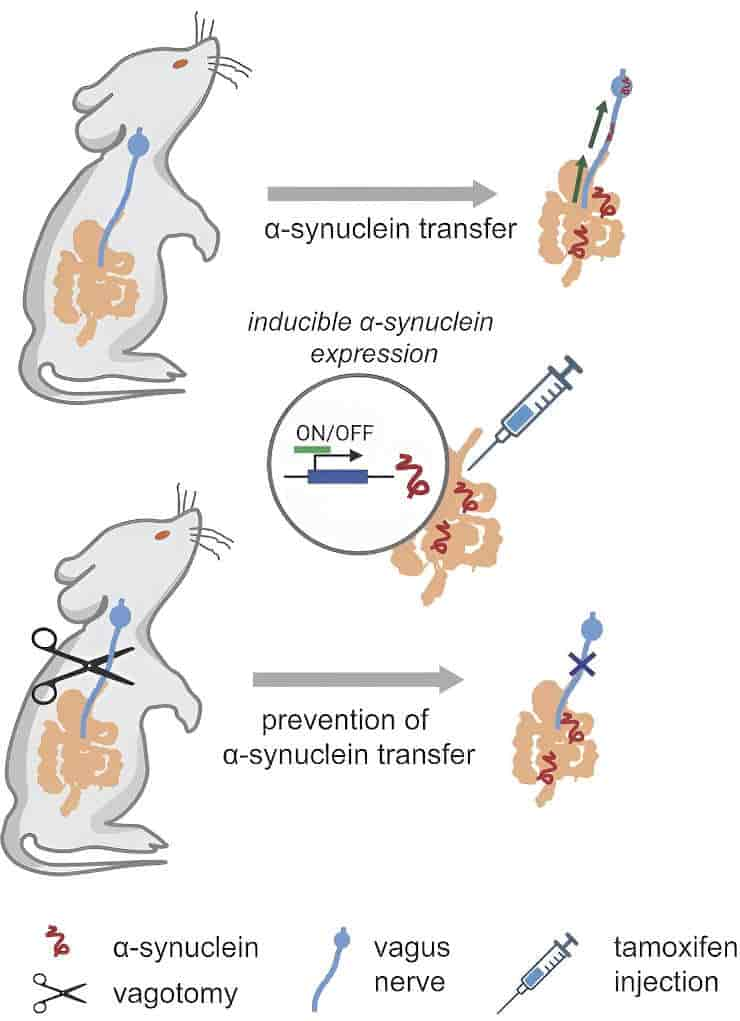
The researchers discovered that enteroendocrine cells transport alpha synuclein from gut mucosal cells to the brainstem via the vagus nerve, the body’s superhighway connecting the gut and brain.
“We hypothesize that something in the gut is corrupting ⍺-synuclein, causing it to misfold. Whether this is toxicants or some other exposure, we don’t know. But we have demonstrated here that there is a route for pathologically misfolded ⍺-synuclein to be transported from enteroendocrine cells to the brain, where they can aggregate to form Lewy body deposits,”
Liddle said.
According to Liddle, the study team was able to stop the spread of -synuclein in the rats by cutting the vagus nerve. The discovery lays the groundwork for developing medicines that could either inhibit the transport system or restore the disrupted gut-brain connection.
The study notes that enteroendocrine cells interact with glia and form connections with enteric neurons, in addition to the vagus nerve. It is plausible that alpha-synuclein can travel to enteric glia, providing a potential route for alpha-synuclein spread. It is still unknown what function glia may play in the spread of alpha-synuclein to enteric nerves, particularly the vagus nerve, which extends outside the gut.
Abstract
Epidemiological and histopathological findings have raised the possibility that misfolded α-synuclein protein might spread from the gut to the brain and increase the risk of Parkinson’s disease. Although past experimental studies in mouse models have relied on gut injections of exogenous recombinant α-synuclein fibrils to study gut-to-brain α-synuclein transfer, the possible origins of misfolded α-synuclein within the gut have remained elusive. We recently demonstrated that sensory cells of intestinal mucosa express α-synuclein. Here, we employed mouse intestinal organoids expressing human α-synuclein to observe the transfer of α-synuclein protein from epithelial cells in organoids to cocultured nodose neurons devoid of α-synuclein. In mice expressing human α-synuclein, but no mouse α-synuclein, α-synuclein fibril-templating activity emerged in α-synuclein–seeded fibril aggregation assays in intestine, vagus nerve, and dorsal motor nucleus. In newly engineered transgenic mice that restrict pathological human α-synuclein expression to intestinal epithelial cells, α-synuclein fibril-templating activity transfered to the vagus nerve and dorsal motor nucleus. Subdiaphragmatic vagotomy prior to induction of α-synuclein expression in intestinal epithelial cells effectively protected the hindbrain from emergence of α-synuclein fibril-templating activity. Overall, these findings highlight a potential non-neuronal source of fibrillar α-synuclein protein that might arise in gut mucosal cells.
Reference:
- Rashmi Chandra et al. Gut mucosal cells transfer α-synuclein to the vagus nerve. JCI Insight. 2023; 8(23):e172192 DOI: 10.1172/jci.insight.172192
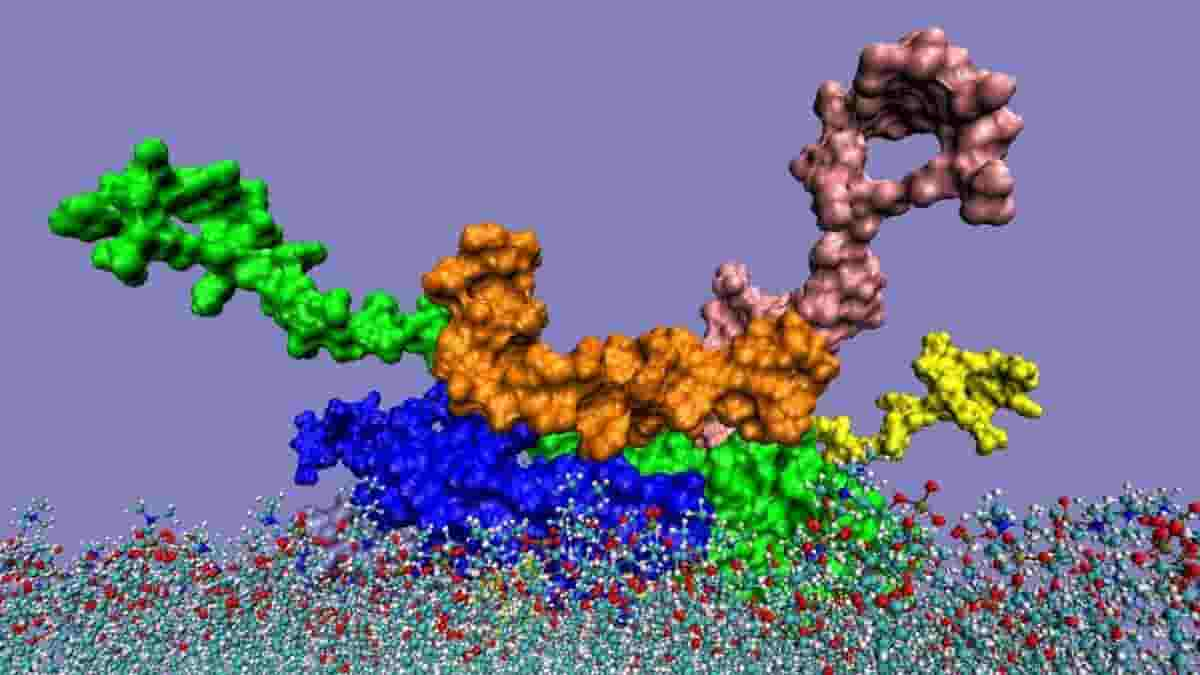
Top image: interaction of the alpha-synuclein pentamer with amyloid-beta 1-42 peptide (orange) when penetrating to the cell membrane. Credit: I. F. Tsigelny, Y. Sharikov, E. Masliah, Agonnne National Laboratory