In a first-of-its-kind study, researchers found that lost brain protection systems and overactive inflammation may contribute to the risk of suicide. The results provide additional justification for investigating the potential of anti-inflammatory drugs as a preventive measure, particularly in scenarios of early suicidal ideation detection.
“As suicide rates continue to rise, we must develop additional evidence-based strategies to address all the factors that contribute to suicide risk. Our study pinpoints several key changes in the brain that one day could be targeted for treatment with the goal of reducing risk and saving lives,”
said the Van Andel Institute’s Lena Brundin, M.D., Ph.D., leader of the study. Columbia University Department of Psychiatry’s J. John Mann, M.D., and Western Michigan University Homer Stryker M.D. School of Medicine’s Eric Achtyes, M.D., M.S. also co-led the study.
Finding Key Molecular Differences
A combination of psychological, social, and biological variables contribute to suicidal behavior. Previous research, including results by Brundin, Mann, and Achtyes, suggests that chronic inflammation may generate a toxic imbalance that affects brain chemistry and increases the risk of suicide.
The new findings expand on previous research by identifying key molecular differences that drive inflammation and may contribute to suicidal behavior.
The brains of 29 persons who died by suicide were compared to the brains of 32 people who died from other causes by the research team. The study’s suicide victims were mostly free of antidepressant and antipsychotic medicines, allowing the researchers to observe suicide-related molecular changes that would otherwise be hidden.
“Our goal is to prevent suicide by better understanding the brain function associated with it. We focused on the brain because that’s where the biological processes that affect mood, suicidal ideation and intent, and decision-making reside. This study enabled us to see the brain at the moment of greatest risk and pinpoint biological markers of that risk,”
Mann said.
Gene Methylation and Transcriptomics
Overall, the researchers discovered increased inflammation as well as decreased activation in brain-protection pathways. Specific changes in the brains of suicide victims include:
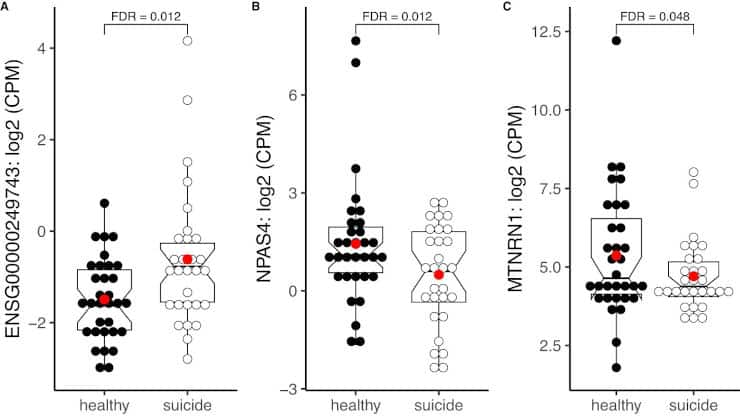
- Less activity in the gene NPAS4, which regulates inflammation and aids in the health of brain cells. This decreased activity promotes inflammation.
- More excitotoxicity, an inflammatory process contributing to cell death.
- Fewer oligodendrocytes — specialized cells that protect nerve fibers. Evidence suggests that these critical cells may succumb to inflammation-induced damage, leaving nerve fibers exposed.
The study also represents the most thorough analysis to date of integrated gene methylation and transcriptomic data derived from the brains of people who died by suicide.
Gene methylation is the process by which genes are turned “on” or “off” by marking them with specific chemical tags. The study discovered methylation patterns that increased abnormal inflammation in people who died by suicide.
Limitations
One limitation of this study is the brain tissue dissection process, which can result in minor differences in the anatomical region employed for analysis. Because the dissected brain gyri fold in three dimensions, excluding all white matter while still taking the full thickness of the cortical ribbon is impossible.
Another limitation is the DNA methylation analysis, which used the Illumina EPIC array platform, designed to cover 30% of the human methylome. As a result, conclusions drawn from these methylation profiles must be interpreted with caution.
Also, the DNA methylation analysis only detects the CpG sites (DNA areas of cytosine and guanine separated by only one phosphate group) while it has been shown that non-CpG methylation can also play a role in both neurons and glial cells, especially later in life.
Next Steps
Brundin, Mann, and Achtyes are looking for biomarkers — measurable molecules in the blood that connect to suicide risk — along with the current study. They envision a future in which clinicians have a validated blood test to assess suicide risk as well as approved therapeutic techniques to minimize that risk, maybe by addressing inflammation.
Future research will concentrate on better understanding the role of inflammation in suicide risk, searching for biomarkers, and developing strategies to evaluate potential treatment options.
“Clinicians desperately need enhanced ways to identify patients at increased risk of suicide. Detecting patterns in molecular markers to flag those at heightened risk could be a valuable tool for helping individuals who are struggling,”
Achtyes said.
Abstract
Suicide rates have increased steadily world-wide over the past two decades, constituting a serious public health crisis that creates a significant burden to affected families and the society as a whole. Suicidal behavior involves a multi-factorial etiology, including psychological, social and biological factors. Since the molecular neural mechanisms of suicide remain vastly uncharacterized, we examined transcriptional- and methylation profiles of postmortem brain tissue from subjects who died from suicide as well as their neurotypical healthy controls. We analyzed temporal pole tissue from 61 subjects, largely free from antidepressant and antipsychotic medication, using RNA-sequencing and DNA-methylation profiling using an array that targets over 850,000 CpG sites. Expression of NPAS4, a key regulator of inflammation and neuroprotection, was significantly downregulated in the suicide decedent group. Moreover, we identified a total of 40 differentially methylated regions in the suicide decedent group, mapping to seven genes with inflammatory function. There was a significant association between NPAS4 DNA methylation and NPAS4 expression in the control group that was absent in the suicide decedent group, confirming its dysregulation. NPAS4 expression was significantly associated with the expression of multiple inflammatory factors in the brain tissue. Overall, gene sets and pathways closely linked to inflammation were significantly upregulated, while specific pathways linked to neuronal development were suppressed in the suicide decedent group. Excitotoxicity as well as suppressed oligodendrocyte function were also implicated in the suicide decedents. In summary, we have identified central nervous system inflammatory mechanisms that may be active during suicidal behavior, along with oligodendrocyte dysfunction and altered glutamate neurotransmission. In these processes, NPAS4 might be a master regulator, warranting further studies to validate its role as a potential biomarker or therapeutic target in suicidality.
Reference:
- Sha, Q., Fu, Z., Escobar Galvis, M.L. et al. Integrative transcriptome- and DNA methylation analysis of brain tissue from the temporal pole in suicide decedents and their controls. Mol Psychiatry (2023). doi: 10.1038/s41380-023-02311-9
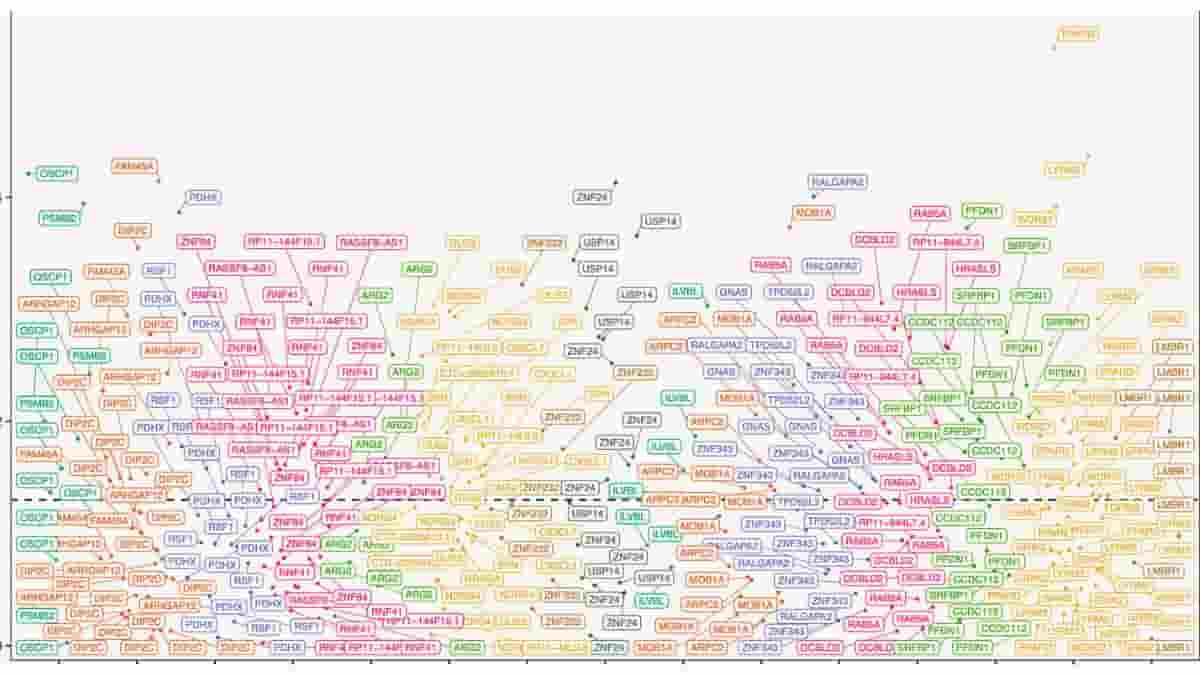
Top Image: Manhattan plot of differential methylation regions (DMRs) on chromosomes. DMR analysis identified 40 regions that were significantly differentially methylated between suicide and control group. Credit: Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02311-9