A new study confirms that SARS-CoV-2 is capable of infecting human neurons in vitro and migrating into axons, the nerve cell projections that carry information.
The emergence of many SARS-CoV-2 mutations has resulted in a wide range of clinical profiles and symptoms in patients. For the first time, scientists at the Institut Pasteur and Université Paris Cité revealed in an animal model a feature shared by numerous SARS-CoV-2 variants: the potential to infect the central nervous system.
The neurological symptoms associated with SARS-CoV-2 infection have changed as the virus has evolved and new variants have emerged. Loss of the sense of smell (anosmia) was one of the characteristic symptoms of infection at the outset of the COVID-19 pandemic, however it was less frequently related with infections caused by the omicron/BA.1 variety. Is this variation in symptoms indicative of a greater or lesser affinity of SARS-CoV-2 for the nervous system?
Olfactory Bulb Infection
Scientists from Institut Pasteur and Université Paris Cité used an animal model to show that several types of SARS-CoV-2 viruses can enter and stay in the central nervous system during the early stages of infection. These types include the original strain of the virus that was first found in Wuhan, as well as the gamma, delta, and omicron/BA.1 variants.
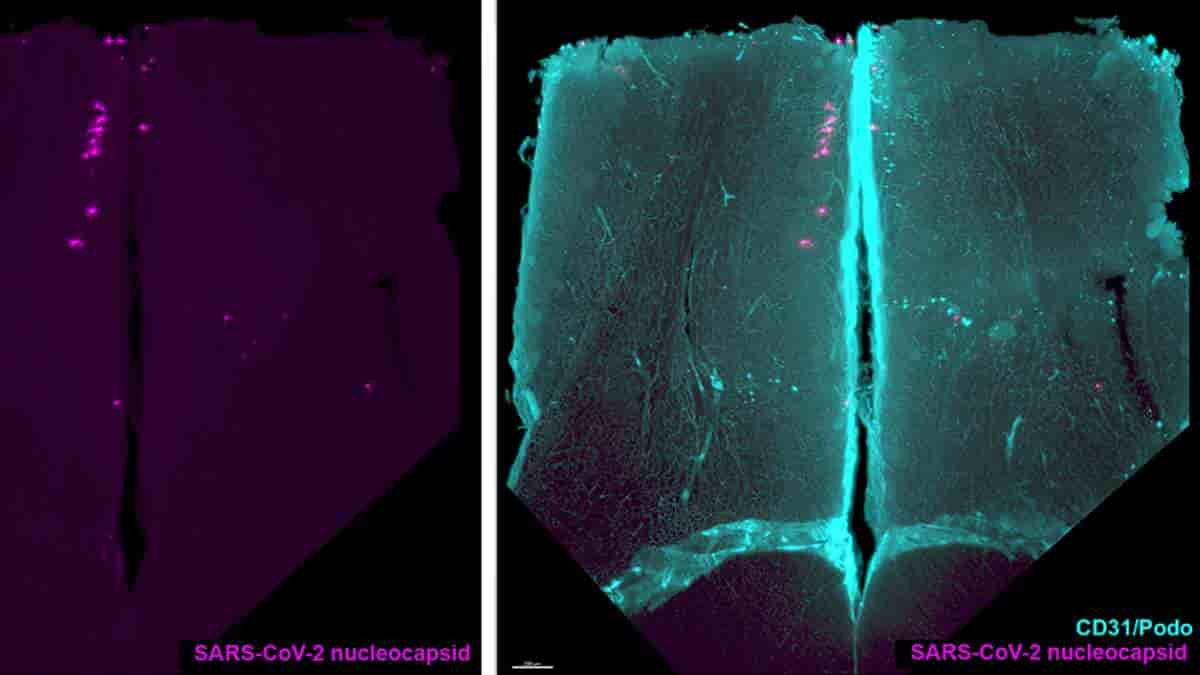
All of these variants spread to the central nervous system and infect the olfactory bulb, a structure in the cranial cavity that processes olfactory information before transmitting it to the cortex, according to the researchers.
“In this study, we demonstrated that infection of the olfactory bulb is common to all variants and not linked to any particular one, nor to any particular clinical manifestation such as anosmia,”
explained first author Guilherme Dias de Melo, researcher in the Institut Pasteur’s Lyssavirus, Epidemiology and Neuropathology Unit.
Asymptomatic Neural Migration
Furthermore, the researchers discovered a genetic sequence linked to anosmia in the ancestral (Wuhan) virus. When this genetic sequence, which encodes the ORF7ab protein, is deleted or truncated — which is the case in certain variants less likely to produce anosmia — the incidence of olfactory loss in infected animals is lower even though the degree of neuronal infection via the olfactory bulbs remains unchanged.
“This suggests that anosmia and neuronal infection are two unrelated phenomena. If we follow this line of reasoning, it is quite possible that even an asymptomatic, and therefore clinically benign, infection is characterized by the spread of the virus in the nervous system,”
says Guilherme Dias de Melo.
The researchers then looked at how SARS-CoV-2 reached the olfactory bulb and observed that neurons appeared to be the ideal route.
Axon Travel
Credit: Nat Commun 14, 4485 (2023). doi: 10.1038/s41467-023-40228-7 CC-BY
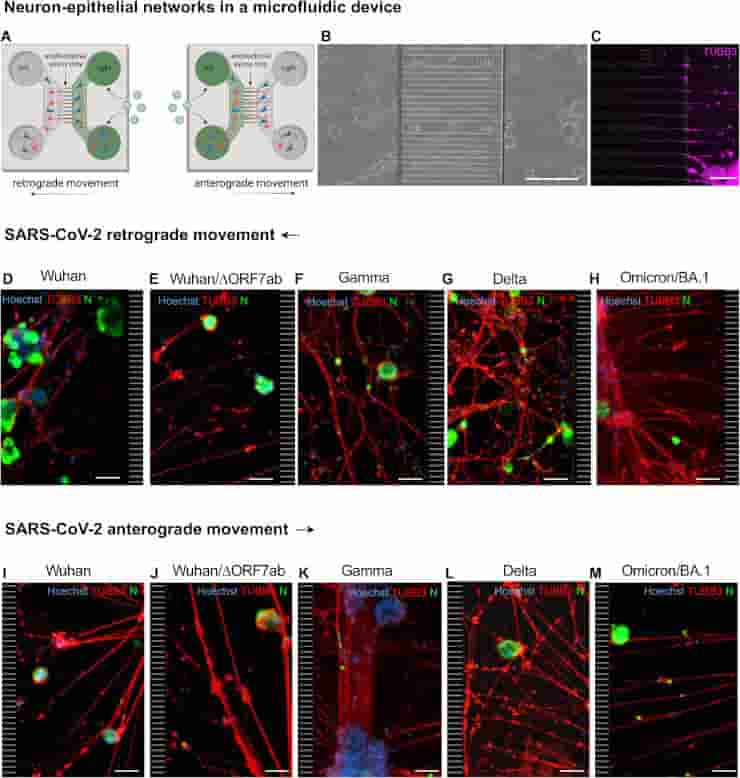
The researchers were able to view human neurons arranged in a certain way thanks to an in vitro microfluidic cell culture technology. The neurons are structured in such a way that the transport of chemicals within the axon may be observed in great detail.
Using this strategy, the researchers discovered that once within the neuron, the virus can migrate in both ways down the axon, either anterogradely (from the cell body to the axon terminals) or retrogradely (from the axons to the cell body).
The virus appears to use the neuron’s physiological capabilities to migrate in both directions. The SARS-CoV-2 variations evaluated by the researchers — the ancestral Wuhan variant, gamma, delta, and omicron/BA.1 – infect neurons in vitro and can move along axons.
“Through this study, we have characterized the neurotropism of SARS-CoV-2. For all the variants studied, brain infection via the olfactory bulb seems to be a common feature of SARS-CoV-2,”
concluded Hervé Bourhy, last author of the study and head of the Institut Pasteur’s Lyssavirus, Epidemiology and Neuropathology Unit.
The next step will be to determine whether the virus can persist in the brain after the acute phase of infection, and whether the presence of the virus can cause persistent inflammation and the symptoms described in cases of long COVID, such as anxiety, depression, and brain fog.
Abstract
Anosmia was identified as a hallmark of COVID-19 early in the pandemic; however, with the emergence of variants of concern, the clinical profile induced by SARS-CoV-2 infection has changed, with anosmia being less frequent. Here, we assessed the clinical, olfactory and neuroinflammatory conditions of golden hamsters infected with the original Wuhan SARS-CoV-2 strain, its isogenic ORF7-deletion mutant and three variants: Gamma, Delta, and Omicron/BA.1. We show that infected animals develop a variant-dependent clinical disease including anosmia, and that the ORF7 of SARS-CoV-2 contributes to the induction of olfactory dysfunction. Conversely, all SARS-CoV-2 variants are neuroinvasive, regardless of the clinical presentation they induce. Taken together, this confirms that neuroinvasion and anosmia are independent phenomena upon SARS-CoV-2 infection. Using newly generated nanoluciferase-expressing SARS-CoV-2, we validate the olfactory pathway as a major entry point into the brain in vivo and demonstrate in vitro that SARS-CoV-2 travels retrogradely and anterogradely along axons in microfluidic neuron-epithelial networks.
Reference:
- de Melo, G.D., Perraud, V., Alvarez, F. et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Nat Commun 14, 4485 (2023). doi: 10.1038/s41467-023-40228-7
Top Image: Representative olfactory bulb section stained for SARS-CoV-2 nucleocapsid and for podoplanin (CD31/Podo) to identify the vascular compartment. Note presence of SARS-CoV-2 in olfactory bulb neurons, with no macroscopic alterations in the vascular network of the olfactory bulb. Credit: Nat Commun 14, 4485 (2023). doi: 10.1038/s41467-023-40228-7 CC-BY