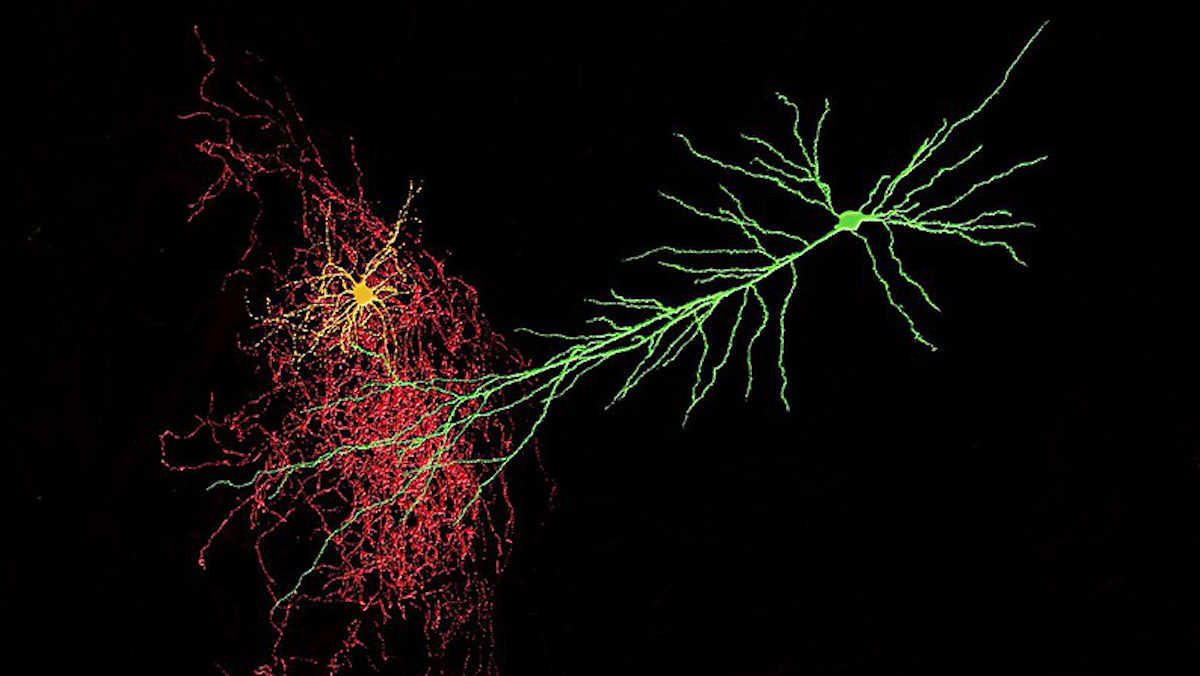
A mechanism by which neurons can regulate their sensitivity to incoming signals autonomously has been discovered in a new study led by the University of Bonn. The researchers, including those from the German Center for Neurodegenerative Diseases and the Max Planck Institute for Neurobiology of Behavior, investigated pyramidal neurons – nerve cells that also play a role in vision, hearing and touch.
A stimulus first travels to the thalamus, a structure deep within the brain’s center. It is then transported to the cerebral cortex, where it is further processed.
“The neurons in the cerebral cortex are stimulated by the signals from the thalamus to generate action potentials. These are short voltage pulses that are then transmitted to other sites in the brain. For that to work well, the neurons have to adjust to the intensity of the excitatory signal,”
explained Prof. Dr. Heinz Beck from the Institute of Experimental Epileptology and Cognition Research at the University Hospital Bonn.
If the incoming stimuli were particularly potent, for instance, neurons would need to lower their sensitivity.
SLK Enzyme
Researchers have now learned that a particular enzyme known as Ste20-like kinase (SLK) contributes to this action.
“It enables neurons to individually calibrate their own excitability,”
said Beck.
Anyone who has ever used a cell phone to send a voice message is aware of how important volume is. A recording that is distorted and hazy is the result of shouting into the microphone. However, whispering is also a bad idea because the result is too quiet and hard to understand.
That is why sound engineers ensure that every concert and talk show has perfect sound: they adjust the gain of each microphone to match the input signal.
The neurons in the brain are like this too. That is where the SLK enzyme comes in.
It’s similar to not having a sound engineer: the microphones would automatically adjust their sensitivity so that the recording is neither too quiet nor overly amplified.
Interneuron Adjustments
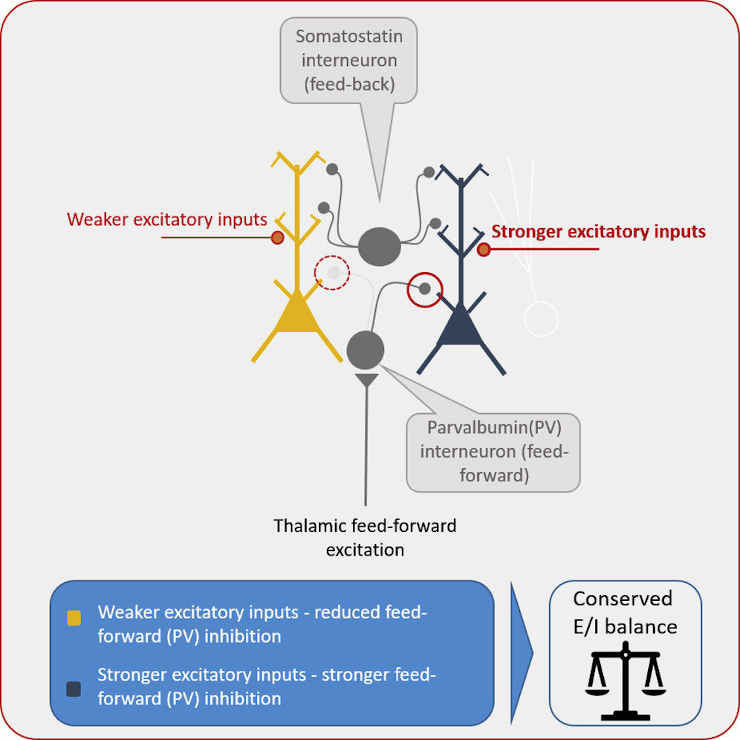
Special nerve cells called interneurons play an essential role in this mechanism. Interneurons transmit inhibitory action potentials to neurons that are excited. In a sense, they adjust the dial that decreases their sensitivity.
“The SLK now determines how much this regulator can be adjusted by the interneurons, that is, how strong their inhibitory effect is,”
said Dr. Pedro Royero from Beck’s research group.
Two distinct types of interneurons exist.
Some neurons are activated directly by thalamic impulses. They already inhibit the neurons, which the thalamus is simultaneously stimulating.
In contrast, another type of inhibitory neurotransmitter is only activated by the activity of neurons in the cerebral cortex, i.e. the neurons they are intended to inhibit. Consequently, they are involved in a negative feedback loop.
“Interestingly, the SLK is not active in this feedback inhibition, but only in the first case,”
Royero noted.
Genetic Activation
Additionally, the researchers demonstrated that certain genes are activated during sensitivity adjustment.
Now, they wish to investigate their role in the procedure in greater depth. This is also fascinating because the equilibrium between excitation and inhibition is crucial for brain function.
This is evident, for instance, in epilepsy. Large regions of nerve cells are overstimulated, resulting in the characteristic seizures.
In fact, research indicates that in some epilepsy patients, SLK levels in neurons are lower than normal. Consequently, perhaps the study will also contribute to a greater comprehension of these disease mechanisms.
Reference:
Pedro Royero, Anne Quatraccioni, Rieke Früngel, Mariella Hurtado Silva, Arco Bast, Thomas Ulas, Marc Beyer, Thoralf Opitz, Joachim L. Schultze, Mark E. Graham, Marcel Oberlaender ,Albert Becker, Susanne Schoch, Heinz Beck. Circuit-selective cell-autonomous regulation of inhibition in pyramidal neurons by Ste20-like kinase. Cell Reports Volume 41, Issue10, 111757, December 6, 2022