A multinational group of researchers has demonstrated that injecting a particular kind of stem cell into the brains of people with progressive multiple sclerosis (MS) is safe, well-tolerated, and produces a long-lasting effect that seems to shield the brain from additional damage. The work, led by scientists at the University of Cambridge, the University of Milan Bicocca, and the Hospital Casa Sollievo della Sofferenza (Italy), is an important step toward developing an advanced cell therapy treatment for progressive MS.
While treatments exist to reduce the severity and frequency of relapses, two-thirds of MS patients still progress into a debilitating secondary progressive phase of disease within 25-30 years of diagnosis, where disability worsens steadily. In multiple sclerosis, the body’s own immune system attacks and damages myelin, the protective sheath around nerve fibers, causing disruption to messages sent around the brain and spinal cord.
More than 2 million people live with MS around the world currently.
Transplanting Brain Stem Cells
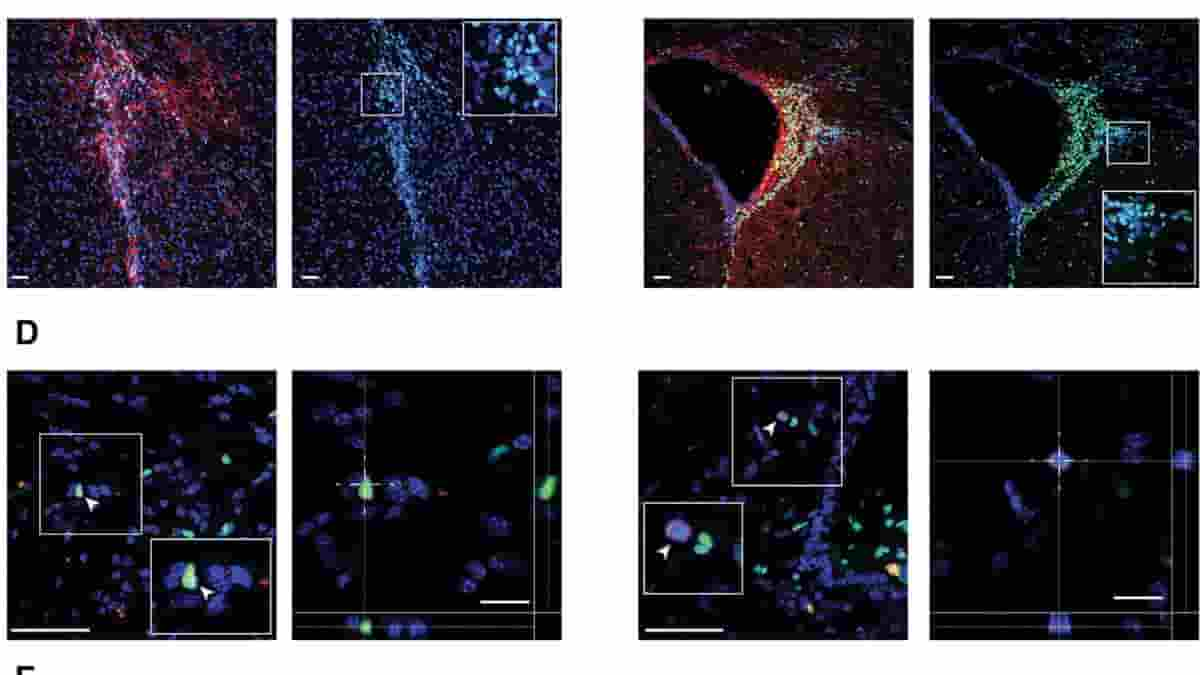
Key immune cells involved in the demyelination process are macrophages (literally ‘big eaters’), which ordinarily attack and rid the body of unwanted intruders. Microglial cells are a type of macrophage found throughout the brain and spinal cord. They affect the central nervous system (CNS) in progressive forms of MS, producing chronic inflammation and nerve cell destruction.
Recent advances have increased hopes that stem cell therapies will be able to help mitigate this damage. These procedures entail the transplantation of stem cells, the body’s ‘master cells,’ which can be programmed to develop into nearly any type of cell within the body.
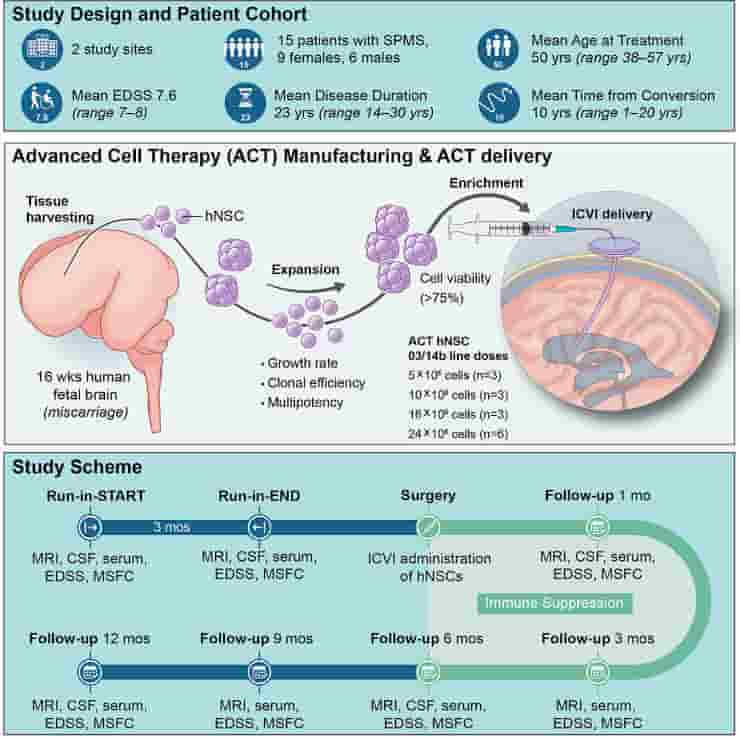
Previous work from the Cambridge team has shown in mice that skin cells re-programmed into brain stem cells, transplanted into the central nervous system, can help reduce inflammation and may be able to help repair damage caused by MS. Scientists have now completed a first-in-human, early-stage clinical trial that involved injecting neural stem cells directly into the brains of 15 patients with secondary MS recruited from two Italian hospitals.
No Cognitive Ability Deterioration
The trial was conducted by teams at the University of Cambridge, Milan Bicocca and the Hospitals Casa Sollievo della Sofferenza and S. Maria Terni (IT) and Ente Ospedaliero Cantonale (Lugano, Switzerland) and the University of Colorado (U.S.). The stem cells were derived from cells extracted from brain tissue of a single miscarried fetal donor.
The Italian team had previously demonstrated that it would be possible to generate a virtually limitless supply of these stem cells from a single donor — and in the future, it may be possible to derive these cells directly from the patient, assisting in overcoming practical issues associated with the use of allogeneic fetal tissue.
The team followed the patients for 12 months, during which time they observed no treatment-related deaths or serious adverse events. While some side effects were observed, all were either temporary or reversible.
All of the patients had substantial levels of disability at the outset of the trial — most needed a wheelchair, for example — but no one showed any increase in disability or worsening of symptoms during the 12-month follow-up period. None of the patients reported relapse symptoms, nor did their cognitive ability deteriorate significantly during the trial.
Reduced Inflammation
Overall, say the researchers, the results indicate substantial stability of the disease, without signs of progression, though the high levels of disability at the start of the trial make this difficult to confirm.
The researchers examined a subset of individuals for changes in brain tissue volume linked with illness progression. The researchers discovered that the higher the dose of injected stem cells, the smaller the decline in brain volume over time. They believe this may be because the stem cell transplant reduced inflammation.
The team also looked for signs that the stem cells were having a neuroprotective effect — that is, protecting nerve cells from further damage. Their previous work showed how tweaking metabolism — how the body produces energy — can in turn reprogram microglia from ‘bad’ to ‘good.
Fatty Acid Levels
In this new study, they looked at how the brain’s metabolism changes after the treatment. They measured changes in the fluid around the brain and in the blood over time and found certain signs that are linked to how the brain processes fatty acids.
These indicators were linked to how effectively the treatment works and how the disease progresses. The bigger the dose of stem cells, the higher the fatty acid levels, which lasted during the 12-month period.
“We desperately need to develop new treatments for secondary progressive MS, and I am cautiously very excited about our findings, which are a step towards developing a cell therapy for treating MS,”
Professor Stefano Pluchino from the University of Cambridge, who co-led the study, said.
The authors recognize that the study has limitations. It was only a small study and there may have been confounding effects from the immunosuppressant drugs, for example. However, because the treatment was safe and its effects lasted for 12 months, the researchers can now move on to the next stage of clinical trials.
“These results show that special stem cells injected into the brain were safe and well-tolerated by people with secondary progressive MS. They also suggest this treatment approach might even stabilize disability progression. We’ve known for some time that this method has the potential to help protect the brain from progression in MS,”
said Caitlin Astbury, Research Communications Manager at the MS Society.
Abstract
We report the analysis of 1 year of data from the first cohort of 15 patients enrolled in an open-label, first-in-human, dose-escalation phase I study (ClinicalTrials.gov: NCT03282760, EudraCT2015-004855-37) to determine the feasibility, safety, and tolerability of the transplantation of allogeneic human neural stem/progenitor cells (hNSCs) for the treatment of secondary progressive multiple sclerosis.
Participants were treated with hNSCs delivered via intracerebroventricular injection in combination with an immunosuppressive regimen. No treatment-related deaths nor serious adverse events (AEs) were observed. All participants displayed stability of clinical and laboratory outcomes, as well as lesion load and brain activity (MRI), compared with the study entry. Longitudinal metabolomics and lipidomics of biological fluids identified time- and dose-dependent responses with increased levels of acyl-carnitines and fatty acids in the cerebrospinal fluid (CSF). The absence of AEs and the stability of functional and structural outcomes are reassuring and represent a milestone for the safe translation of stem cells into regenerative medicines.
Reference:
- Maurizio A. Leone, Maurizio Gelati, Daniela C. Profico. Phase I clinical trial of intracerebroventricular transplantation of allogeneic neural stem cells in people with progressive Multiple Sclerosis. Cell Stem Cell (2023). DOI: 10.1016/j.stem.2023.11.001