Nerve cells in the brain require an immense quantity of energy to maintain their connections for communication with other nerve cells and to survive. Energy production is impaired in Alzheimer’s disease, and synaptic connections eventually deteriorate and fail, resulting in the loss of new memories.
A Scripps Research team has now identified the energetic reactions in brain cells that malfunction and lead to neurodegeneration. Researchers used a small molecule to fix the problem in the mitochondria, the cell’s main energy producers. This allowed many neuron-to-neuron connections to be successfully restored in nerve cell models made from stem cells from Alzheimer’s patients.
These findings highlight that improving mitochondrial metabolism could be a promising therapeutic target for Alzheimer’s and related disorders.
“We thought that if we could repair metabolic activity in the mitochondria, maybe we could salvage the energy production. In using human neurons derived from people with Alzheimer’s, protecting the energy levels was sufficient to rescue a large number of neuronal connections,”
said senior author Stuart Lipton, MD, PhD, clinical neurologist and Co-Director of the Neurodegeneration New Medicines Center at Scripps Research.
SNO-Storm
An aberrant tag consisting of nitrogen (N) and oxygen (O) atoms attached to a sulfur (S) atom, which collectively constitutes the dysfunctional “SNO” enzyme, was identified by Lipton and his team as the cause of an obstruction in energy-generating enzymes in the new study. The team established that a virtual “SNO-Storm” of these S-nitrosylation reactions took place in the neurons of Alzheimer’s disease patients’ brains.
The “SNO-tag” on energy enzymes was initially identified by Lipton and his colleagues through a comparative analysis of human brains obtained from autopsies of individuals with Alzheimer’s disease and those without brain disease. Following this, the scientists produced nerve cells from stem cells extracted from skin biopsies of individuals who, with or without, possessed a genetic mutation associated with Alzheimer’s disease.
Then, using a set of metabolic labels and an oxygen-measuring device, they figured out how much energy cells were making and found that Alzheimer’s nerve cells were lacking in certain ways compared to control cells.
Missing Succinate Molecules
The neuronal Krebs cycle — the cellular process in mitochondria that generates the majority of ATP, an essential molecular energy source — was discovered to be disrupted by the researchers. The group identified a bottleneck (or obstruction) in the process of succinate formation, a crucial molecule that is responsible for a significant portion of the subsequent ATP production.
The obstruction hindered the mitochondria’s capacity to generate the energy required for the maintenance of neurons and their extensive interconnections, as observed in the study.
The researchers postulated that by providing a portion of the absent succinate molecules, it would be possible to reinstate energy generation—in essence, to restart the stagnant mitochondrial Krebs cycle. Since succinate is difficult to enter and exit cells, an analog that could more easily traverse nerve cell membranes was utilized. By preventing further synaptic degeneration and restoring up to 75% of the lost synapses, the strategy was successful.
“Succinate is not a compound that people can now take as a treatment, but it’s proof-of-principle that you can re-energize the Krebs cycle. The beauty of the study is that we were able to show this in living nerve cells derived from Alzheimer’s patients, but we still have to come up with a much better compound in order to develop an effective drug for humans to take,”
said Lipton.
Potential Therapeutic Target
Lipton, who has developed FDA-approved Alzheimer’s disease medications in the past, including Namenda®, recognizes that a substantial amount of additional research is required to develop a human-safe and efficacious energy-preserving drug.
His research group will persist in investigating the mitochondrial Krebs cycle as a prospective therapeutic target with the ultimate goal of reinstating neuronal connectivity in Alzheimer’s patients. This would effectively halt the progression of the disease and improve cognitive function.
The study does have some limitations, the authors concede. The study is based on findings in postmortem human brains from Alzheimer’s patients, patients and controls. Although postmortem times were short (in the range of a few hours), there is always the possibility that agonal changes could skew the findings.
Abstract
In Alzheimer’s disease (AD), dysfunctional mitochondrial metabolism is associated with synaptic loss, the major pathological correlate of cognitive decline. Mechanistic insight for this relationship, however, is still lacking. Here, comparing isogenic wild-type and AD mutant human induced pluripotent stem cell (hiPSC)-derived cerebrocortical neurons (hiN), evidence is found for compromised mitochondrial energy in AD using the Seahorse platform to analyze glycolysis and oxidative phosphorylation (OXPHOS). Isotope-labeled metabolic flux experiments revealed a major block in activity in the tricarboxylic acid (TCA) cycle at the α-ketoglutarate dehydrogenase (αKGDH)/succinyl coenzyme-A synthetase step, metabolizing α-ketoglutarate to succinate. Associated with this block, aberrant protein S-nitrosylation of αKGDH subunits inhibited their enzyme function. This aberrant S-nitrosylation is documented not only in AD-hiN but also in postmortem human AD brains versus controls, as assessed by two separate unbiased mass spectrometry platforms using both SNOTRAP identification of S-nitrosothiols and chemoselective-enrichment of S-nitrosoproteins. Treatment with dimethyl succinate, a cell-permeable derivative of a TCA substrate downstream to the block, resulted in partial rescue of mitochondrial bioenergetic function as well as reversal of synapse loss in AD-hiN. These findings have therapeutic implications that rescue of mitochondrial energy metabolism can ameliorate synaptic loss in hiPSC-based models of AD.
Reference:
- A. Y. Andreyev, H. Yang, P.-T. Doulias, N. Dolatabadi, X. Zhang, M. Luevanos, M. Blanco, C. Baal, I. Putra, T. Nakamura, H. Ischiropoulos, S. R. Tannenbaum, S. A. Lipton, Metabolic Bypass Rescues Aberrant S-nitrosylation-Induced TCA Cycle Inhibition and Synapse Loss in Alzheimer’s Disease Human Neurons. Adv. Sci. 2024, 2306469. Doi: 10.1002/advs.202306469
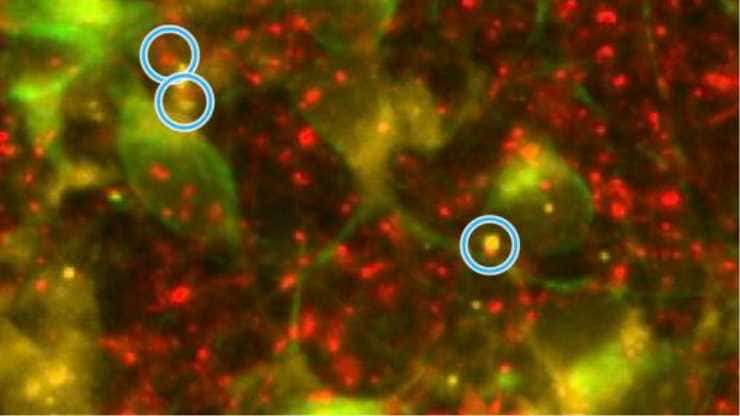
Image: Alzheimer’s nerve cells manifest a decrease in the connections between nerve cells, called synapses, shown here within the blue circles. Half the synapse is marked with a red fluorescent stain and the other half with a yellow stain. Credit: Scripps Research