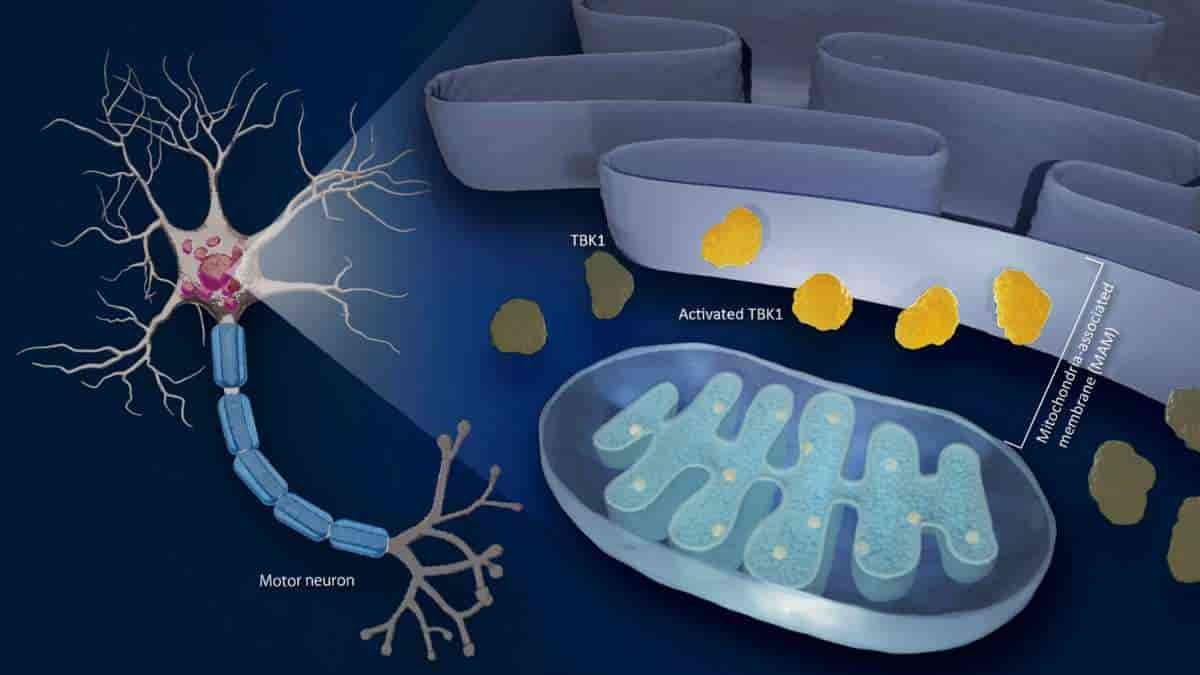
Nagoya University researchers have identified a link between the progression of amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, and the disruption of mitochondria-associated membranes (MAM), the cell’s contact point between the mitochondria and the endoplasmic reticulum (ER). The finding provides important data about the mechanisms behind this neurodegenerative condition.
Motor neurons are targeted by the complex disease ALS. The ER, a complex membrane network that facilitates protein synthesis, metabolism, and calcium storage, and mitochondria, the energy-generating cells of the body, have been implicated in prior research.
The MAM is where the endoplasmic reticulum interfaces with mitochondria. Although both mitochondria and ER abnormality, especially at the mitochondria-associated membrane, play a role in the progression of the disease, it is not clear how.
TANK-binding Kinase 1
TANK-binding kinase 1 (TBK1), an enzyme that is essential for numerous biological processes such as cellular protein clearance and inflammation, is one potential agent. Its disruption plays a crucial role in the pathogenesis of numerous diseases. While ALS is attributed to mutations in the TBK1 gene, the precise mechanism by which TBK1 dysfunctions contribute to its progression remains unknown.
A team led by Koji Yamanaka of Nagoya University’s Research Institute of Environmental Medicine, in collaboration with Masahisa Katsuno of the Graduate School of Medicine, found that brain and spinal cord tissues in ALS patients, as well as mice with a disrupted mitochondria-associated membrane, showed decreased activation of TBK1.
“TBK1 is crucial for stress response in motor neurons. If we reduce its activity, it will result in reduced tolerance to stressors, leading to neurotoxicity and eventual motor neuron death. This finding is particularly significant because abnormal protein accumulation and the resulting stress cause ALS and other neurodegenerative diseases,”
Seiji Watanabe, the first author of the study, explained.
TBK1 Activity Restoration
In patients with ALS, TBK1 activity declines when MAM is abnormal. Upon administering arsenite, an agent known to inhibit MAM and decrease TBK1 activity, to rodents, the researchers observed the development of motor impairments reminiscent of those observed in individuals with ALS.
“Our study strongly suggests that MAM significantly influences the stress response of motor neurons through TBK1 activation. Our study is consistent with human genetic studies that reported that TBK1 mutations cause ALS. Restoring TBK1 activity emerges as a potential therapeutic strategy to counter ALS, marking a promising direction for future research endeavors.”
Yamanaka added.
By focusing on the TBK1 pathway, the researchers have found a critical foundation for developing new ways to treat ALS and possibly other brain disorders.
“We expect these results to lead to the development of new therapeutic strategies for ALS in the future. In future projects, restoring the TBK1 activity (or function) may be a therapeutic strategy to treat ALS,”
said Yamanaka.
Abstract
The organelle contact site of the endoplasmic reticulum and mitochondria, known as the mitochondria-associated membrane (MAM), is a multifunctional microdomain in cellular homeostasis. We previously reported that MAM disruption is a common pathological feature in amyotrophic lateral sclerosis (ALS); however, the precise role of MAM in ALS was uncovered. Here, we show that the MAM is essential for TANK-binding kinase 1 (TBK1) activation under proteostatic stress conditions. A MAM-specific E3 ubiquitin ligase, autocrine motility factor receptor, ubiquitinated nascent proteins to activate TBK1 at the MAM, which results in ribosomal protein degradation. MAM or TBK1 deficiency under proteostatic stress conditions resulted in increased cellular vulnerability in vitro and motor impairment in vivo. Thus, MAM disruption exacerbates proteostatic stress via TBK1 inactivation in ALS. Our study has revealed a proteostatic mechanism mediated by the MAM–TBK1 axis, highlighting the physiological importance of the organelle contact sites.
Reference:
- Watanabe, S., Murata, Y., Oka, Y., Oiwa, K., Horiuchi, M., Iguchi, Y., Komine, O., Sobue, A., Katsuno, M., Ogi, T., & Yamanaka, K. (2023). Mitochondria-associated membrane collapse impairs TBK1-mediated proteostatic stress response in ALS. Proceedings of the National Academy of Sciences of the United States of America, 120(47), e2315347120. Doi: 10.1073/pnas.2315347120
Image: Reiko Matsushita