The interruption of the body’s circadian rhythm, the internal biological clock that regulates many of our physiological functions, is one of the hallmarks of Alzheimer’s disease.
Almost 80% of persons with Alzheimer’s suffer these symptoms, which include difficulties sleeping and decreasing cognitive function at night. However, this aspect of Alzheimer’s disease is not currently addressed by any available treatments.
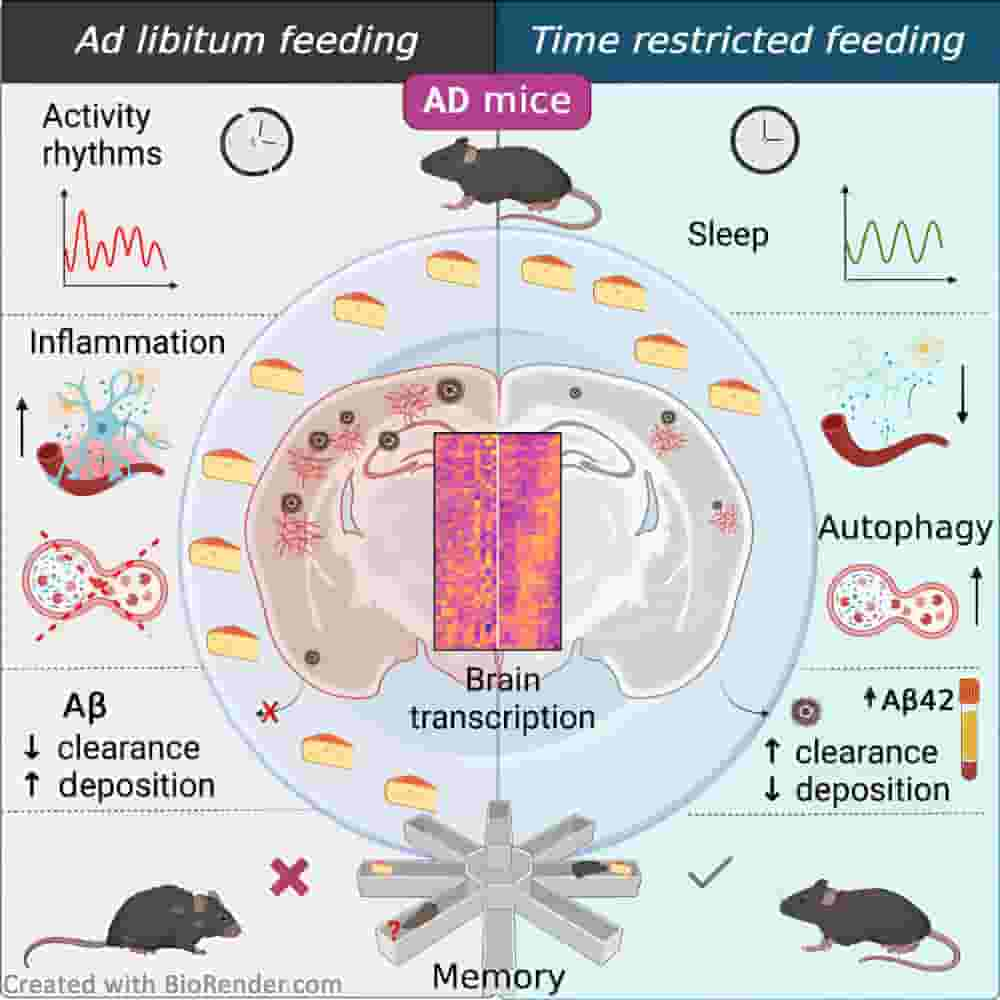
Now, a study from the University of California San Diego School of Medicine found that time-restricted feeding, a type of intermittent fasting focused on limiting the daily eating window without limiting the amount of food consumed, can correct the circadian disruptions seen in Alzheimer’s disease in mice.
Circadian Disruptions and Neurodegeneration
Mice fed on a time-restricted regimen improved their memory and had less amyloid protein buildup in their brain. According to the scientists, the findings will almost certainly lead to a human clinical trial.
“For many years, we assumed that the circadian disruptions seen in people with Alzheimer’s are a result of neurodegeneration, but we’re now learning it may be the other way around—circadian disruption may be one of the main drivers of Alzheimer’s pathology,”
said senior study author Paula Desplats, Ph.D.
Circadian abnormalities are thus a prospective target for future Alzheimer’s treatments, and the findings give proof-of-concept for an easy and accessible method to repair these disruptions.
Looming Health Threat
Alzheimer’s disease, which affects more than 6 million Americans, is often regarded as the country’s most serious looming health threat.
Alzheimer’s patients have a range of circadian rhythm abnormalities, including changes in their sleep/wake cycle, greater cognitive impairment and confusion in the evenings, and trouble sleeping and staying asleep.
“Circadian disruptions in Alzheimer’s are the leading cause of nursing home placement. Anything we can do to help patients restore their circadian rhythm will make a huge difference in how we manage Alzheimer’s in the clinic and how caregivers help patients manage the disease at home,”
said Desplats.
Boosting the Circadian Clock
The management of the daily cycle of eating and fasting is one way to boost the circadian clock, which is a developing strategy for enhancing health outcomes.
The researchers evaluated this strategy on a mouse model of Alzheimer’s disease by restricting the mice’s daily feeding window to six hours. This would equate to approximately 14 hours of daily fasting for humans.
Mice fed on the time-restricted schedule had better memory, were less hyperactive at night, followed a more consistent sleep schedule, and experienced fewer disturbances while sleeping than control mice, who were given food at all times. The time-restricted feeding schedule was able to lessen the behavioural signs of Alzheimer’s disease, as shown by the test mice’s improved cognitive scores compared to those of the control mice.
Molecular Improvement
The researchers also observed molecular improvements in the rodents. Multiple genes associated with Alzheimer’s disease and neuroinflammation were differentially expressed in mice fed on a restricted schedule, scientists found.
In addition, they discovered that the dietary schedule reduced the accumulation of amyloid protein in the brain. Amyloid deposits are among the most recognizable characteristics of Alzheimer’s disease.
The fact that the time-restricted feeding schedule could alter the course of Alzheimer’s in the mice significantly gives the researchers hope that the findings will be simple to apply in the clinic, especially since the new treatment strategy relies on a change in lifestyle rather than the use of medication.
“Time-restricted feeding is a strategy that people can easily and immediately integrate into their lives. If we can reproduce our results in humans, this approach could be a simple way to dramatically improve the lives of people living with Alzheimer’s and those who care for them,”
said Desplats.
Abstract
“Circadian disruptions impact nearly all people with Alzheimer’s disease (AD), emphasizing both their potential role in pathology and the critical need to investigate the therapeutic potential of circadian-modulating interventions. Here, we show that time-restricted feeding (TRF) without caloric restriction improved key disease components including behavioral timing, disease pathology, hippocampal transcription, and memory in two transgenic (TG) mouse models of AD. We found that TRF had the remarkable capability of simultaneously reducing amyloid deposition, increasing Aβ42 clearance, improving sleep and memory, and normalizing daily transcription patterns of multiple genes, including those associated with AD and neuroinflammation. Thus, our study unveils for the first time the pleiotropic nature of timed feeding on AD, which has far-reaching effects beyond metabolism, ameliorating neurodegeneration and the misalignment of circadian rhythmicity. Since TRF can substantially modify disease trajectory, this intervention has immediate translational potential, addressing the urgent demand for accessible approaches to reduce or halt AD progression.”
Reference:
- Daniel S. Whittaker, Laila Akhmetova, Daniel Carlin, Haylie Romero, David K. Welsh, Christopher S. Colwell, Paula Desplats. Circadian modulation by time-restricted feeding rescues brain pathology and improves memory in mouse models of Alzheimer’s disease. Cell Metabolism, 2023, ISSN 1550-4131
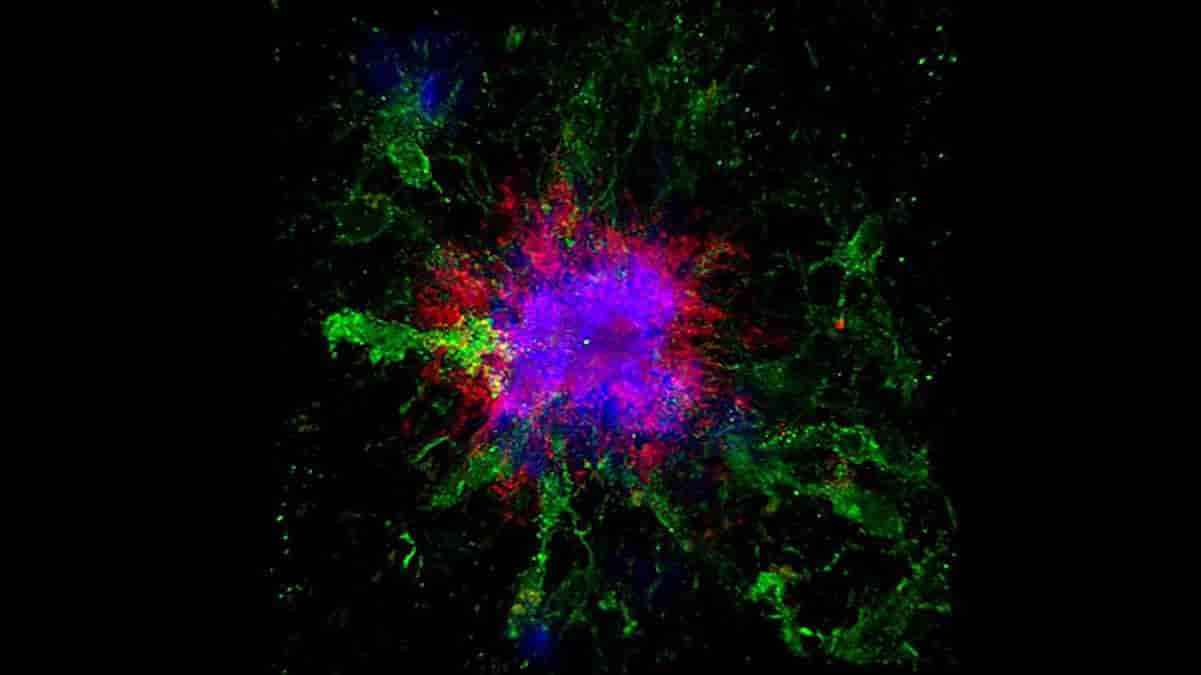
Top Image: confocal microscopy image showing amyloid plaques (blue and red) in the brain of a mouse. Credit: UC San Diego Health Sciences
Last Updated on November 11, 2023