A metabolic rise in a portion of the brain called the hippocampus is an early stage in the development of Alzheimer’s disease, according to Karolinska Institutet researchers. The revelation opens the door to new potential early intervention strategies.
Alzheimer’s disease is the most common type of dementia, affecting approximately 20,000 people in Sweden each year. Researchers have discovered that an increase in metabolic activity in the mitochondria, the cellular power plants, is an early sign of the disease.
“The disease starts to develop 20 years before the onset of symptoms, so it’s important to detect it early, especially given the retardant medicines that are starting to arrive. Metabolic changes can be a diagnostic factor in this,”
said Per Nilsson, associate professor at the Department of Neurobiology, Care Sciences and Society, Karolinska Institutet.
Changes in Metabolism
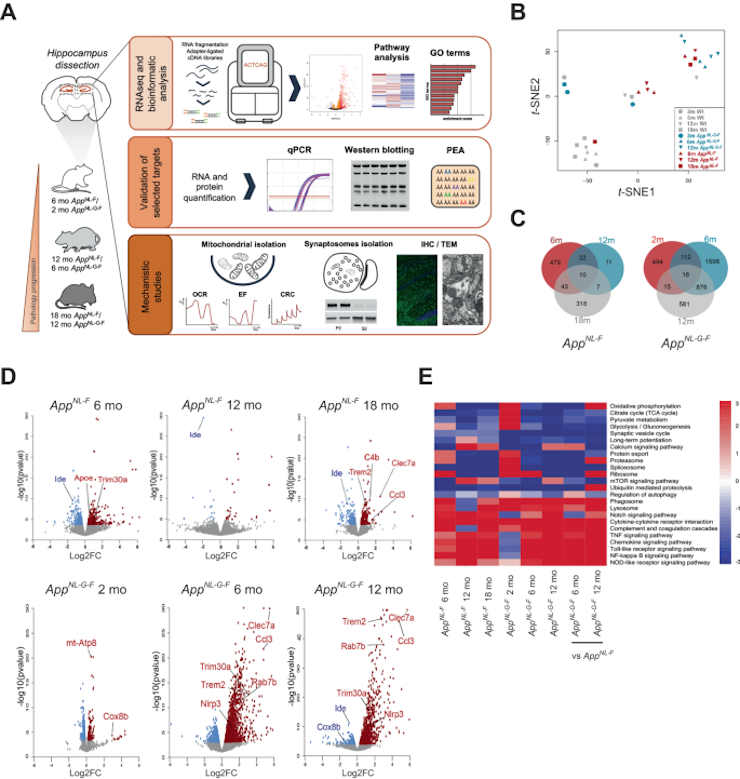
Credit: Molecular Psychiatry (2023). DOI: 10.1038/s41380-023-02289-4
The teams behind the study used mice that developed Alzheimer’s disease pathology in a similar way to humans. The increase in metabolism in young mice was followed by synaptic changes caused by disruption to the cellular recycling system (a process known as autophagy), a finding that was awarded the Nobel Prize in Physiology or Medicine in 2016.
Metabolism in the Alzheimer brain often diminishes over time, contributing to synapse deterioration. This was also observed in older mice that had been infected for a longer period of time.
“Interestingly, changes in metabolism can be seen before any of the characteristic insoluble plaques have accumulated in the brain. The different energy balance tallies with what we’ve seen in images of the Alzheimer brain, but we’ve now detected these changes at an earlier stage,”
Professor Maria Ankarcrona said. The study was conducted in close partnership between both researchers’ groups, who analyzed the part of the mouse brain called the hippocampus, a structure that plays an important part in short-term memory and that is affected early in the pathological process.
Autophagosome Accumulation
The researchers discovered that one of the early stages of the disease is an increase in mitochondrial metabolism by using the technique of RNA sequencing to see which genes are active in the cells of the hippocampus during different stages of the disease.
Using electron microscopy and other techniques, the researchers examined the changes that occurred in the synapses between brain neurons and discovered that vesicles called autophagosomes, which break down spent proteins and metabolize their components, had accumulated in the synapses, disrupting access to functioning proteins.
Now, the researchers are going to look into the part that mitochondria and autophagy play in the development of Alzheimer’s disease in more detail. For example, they will use mice that already have Alzheimer’s disease as an even better model of the brain of an Alzheimer patient.
“These findings highlight the importance of retaining functional mitochondria and normal protein metabolism. Going forward, we’ll be able to do tests on mice to see if new molecules that stabilize mitochondrial and autophagic function can retard the disease,”
said Dr. Nilsson.
Abstract
Accumulation of amyloid β-peptide (Aβ) is a driver of Alzheimer’s disease (AD). Amyloid precursor protein (App) knock-in mouse models recapitulate AD-associated Aβ pathology, allowing elucidation of downstream effects of Aβ accumulation and their temporal appearance upon disease progression. Here we have investigated the sequential onset of AD-like pathologies in AppNL-F and AppNL-G-F knock-in mice by time-course transcriptome analysis of hippocampus, a region severely affected in AD. Strikingly, energy metabolism emerged as one of the most significantly altered pathways already at an early stage of pathology. Functional experiments in isolated mitochondria from hippocampus of both AppNL-F and AppNL-G-F mice confirmed an upregulation of oxidative phosphorylation driven by the activity of mitochondrial complexes I, IV and V, associated with higher susceptibility to oxidative damage and Ca2+-overload. Upon increasing pathologies, the brain shifts to a state of hypometabolism with reduced abundancy of mitochondria in presynaptic terminals. These late-stage mice also displayed enlarged presynaptic areas associated with abnormal accumulation of synaptic vesicles and autophagosomes, the latter ultimately leading to local autophagy impairment in the synapses. In summary, we report that Aβ-induced pathways in App knock-in mouse models recapitulate key pathologies observed in AD brain, and our data herein adds a comprehensive understanding of the pathologies including dysregulated metabolism and synapses and their timewise appearance to find new therapeutic approaches for AD.
Reference:
- Naia, L., Shimozawa, M., Bereczki, E. et al. Mitochondrial hypermetabolism precedes impaired autophagy and synaptic disorganization in App knock-in Alzheimer mouse models. Mol Psychiatry (2023). doi: 10.1038/s41380-023-02289-4
Last Updated on November 11, 2023