Trillions of bacteria, fungi and viruses live inside us, forming what is known as our microbiota. Far from causing problems, these microbes benefit our health in many ways. Most of our microbiota lives in our gut, yet there is increasing evidence that it can influence how our central nervous system works.
Gut microbes produce compounds that prime immune cells to destroy harmful viruses in the brain and nervous system, according to a mouse study published today1.
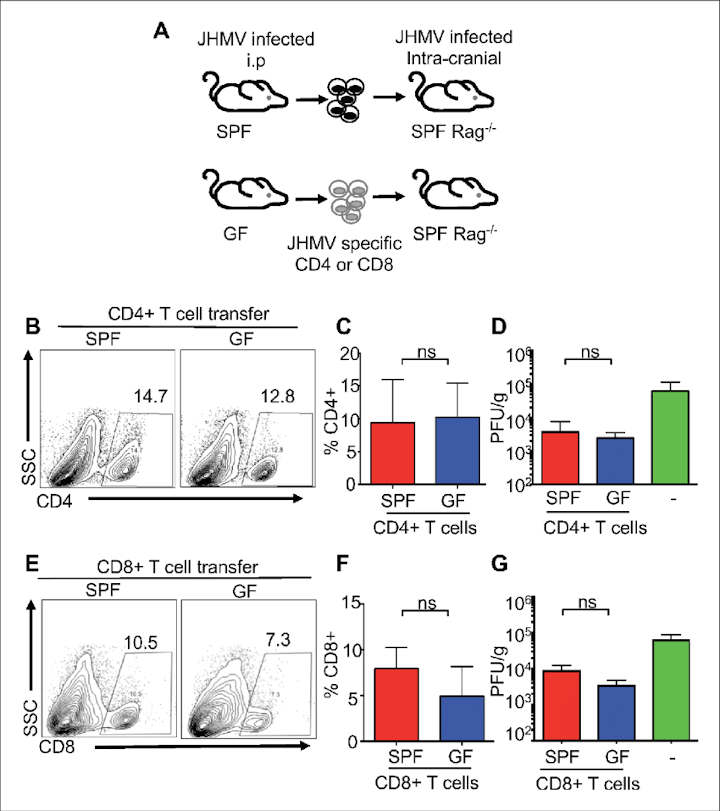
We wanted to investigate whether gut microbes could alter the immune response to a virus in the central nervous system and whether this affects the amount of damage the virus causes,
says one of the lead authors David Garrett Brown, a graduate research assistant in the Department of Pathology at University of Utah Health.
Multiple Sclerosis
A condition that causes progressive damage to nerve cells, multiple sclerosis has become more common over the past several decades. Viral infections in the brain or spinal cord are thought to trigger this disease.
Some scientists believe that changes in the way we eat, increased sanitation or growing antibiotic use may be causing detrimental changes in the helpful bacteria that live within the human body, potentially increasing the risk of multiple sclerosis and other related diseases.
In particular, these communities of microbes could have a role in multiple sclerosis, a disease that emerges when the immune system attacks the insulating sheath which protects neurons, slowly leading to paralysis. What causes multiple sclerosis is still unknown, but scientists believe that a viral infection could trigger the condition.
In the gut, the microbiota helps the immune system to fight off harmful microbes. It is still unclear whether it performs the same role in the central nervous system, and if it can participate in diseases where the immune system harms nerve tissues.
Previous studies in mice have looked into how gut microbes influence the development of illnesses similar to multiple sclerosis, but they did not use the type of live viral infection that is thought to trigger the condition.
Mouse Hepatitis Virus
Garrett Brown and co-lead author Ray Soto looked at the effect of Mouse Hepatitis Virus, a virus that infects cells in the mouse nervous system and causes multiple-sclerosis type symptoms, on two groups of mice: some with normal gut microbes and some that were bacteria-free.
They found that bacteria-free mice had a weak immune response, were unable to eliminate the virus and developed worsening paralysis, while those with normal gut bacteria were better able to fight off the virus.
Mice treated with antibiotics before the onset of disease were unable to defend themselves. They also had fewer immune cells called microglia, which help flag viruses for destruction by other immune cells.
Signals From Microbes
Next, the team identified compounds produced by gut bacteria that might help the microglia. When they administered these helpful compounds to the bacteria-free mice, they saw that the animals were protected from neurologic damage caused by the virus.
Our own genetic background, but also lifestyle changes such as diets, antibiotics or sanitation can influence our microbiota. In parallel, in the past decades there has been an increase in the number of diseases, such as multiple sclerosis, in which the immune system turns against the body.
We’ve shown that gut microbes protect infected mice from paralysis by turning on a specific pathway in central nervous system cells. This suggests that signals from microbes are essential to quickly clear viruses in the nervous system and prevent damage from multiple sclerosis-like diseases. Our results emphasise the importance of maintaining a diverse community of bacteria in the gut, and that interventions to restore this community after taking antibiotics may be necessary,
explains June Round, Associate Professor in the Department of Pathology at University of Utah Health, and a co-senior author alongside Professor Thomas Lane, from the same department. Future work is necessary to understand which of these microbes are protective, and whether they operate during specific timeframes.
Funding for the work came from the Multiple Sclerosis Society, Burroughs Wellcome Fund, National Institutes of Health, and the Ben B. and Iris M. Margolis Foundation
- D Garrett Brown et al. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. eLife (2019). DOI: 10.7554/eLife.47117 ↩︎
Last Updated on November 11, 2022