The link between circadian rhythms — the body’s natural day/night cycles — and synapses was elucidated in a recent study conducted at Boston Children’s Hospital under the direction of Jonathan Lipton, MD, Ph.D. This is the first scholarly contribution to offer a cellular and molecular rationale for the inherent variations in cognition, learning capacity, and memory that occur naturally throughout the day.
“We have known for more than a century that the time of day influences cognition and memory, but until now the mechanisms have been elusive,”
said Lipton, a sleep physician in the Department of Neurology and researcher in the F.M. Kirby Neurobiology Center.
Circadian Protein BMAL1
Lipton and colleagues published in the journal Cell in 2015 an article detailing how BMAL1, a “clock” protein, regulates the timing of protein synthesis in cells. The findings of a new study led by Ilaria Barone, Ph.D., demonstrate that BMAL1 is expressed at specific times of day at brain synapses, controlling the synapses’ reactivity to environmental changes and helping the brain to learn and remember memories.
Lipton thinks that the brain takes advantage of our natural circadian rhythms to conserve energy for when it’s needed.
“Cognitive processes are highly energetically demanding,”
he explained.
While preliminary in nature, the research indicates the potential to enhance cognitive performance in disorders that impact synapses and are influenced by the circadian rhythm, such as fragile X syndrome, Alzheimer’s disease, bipolar disorder, and Parkinson’s disease.
Hippocampus and Beyond
Six years of laborious study and, according to Lipton, over a dozen distinct laboratory techniques went into the production of these results.
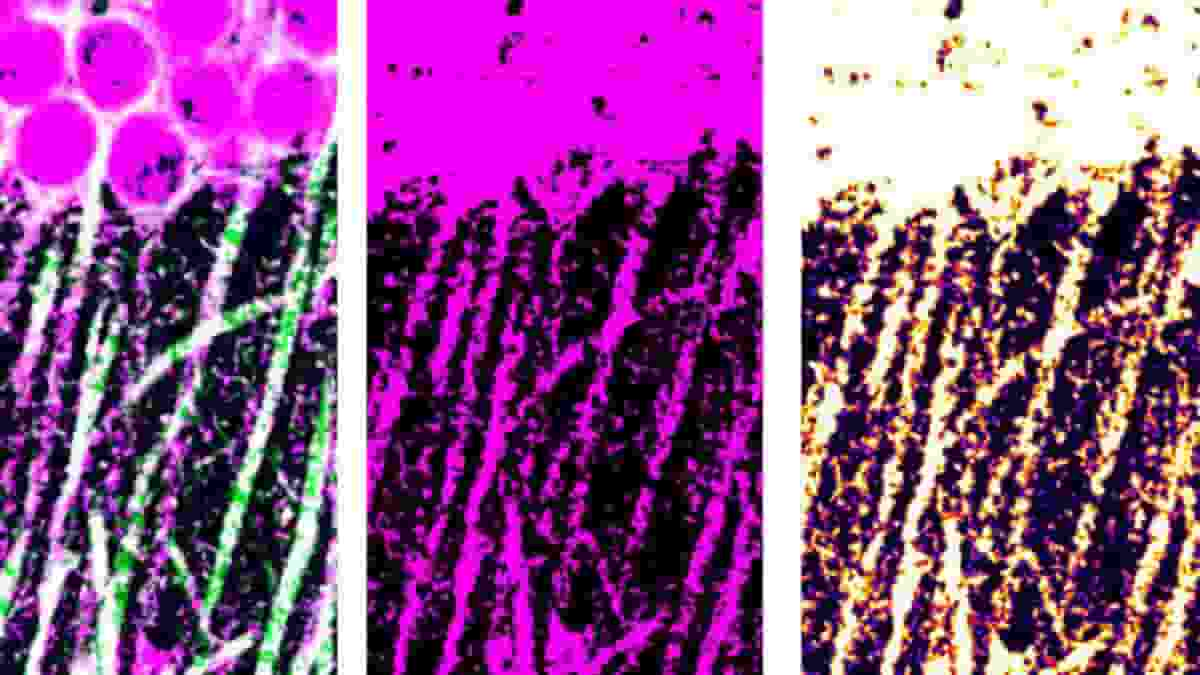
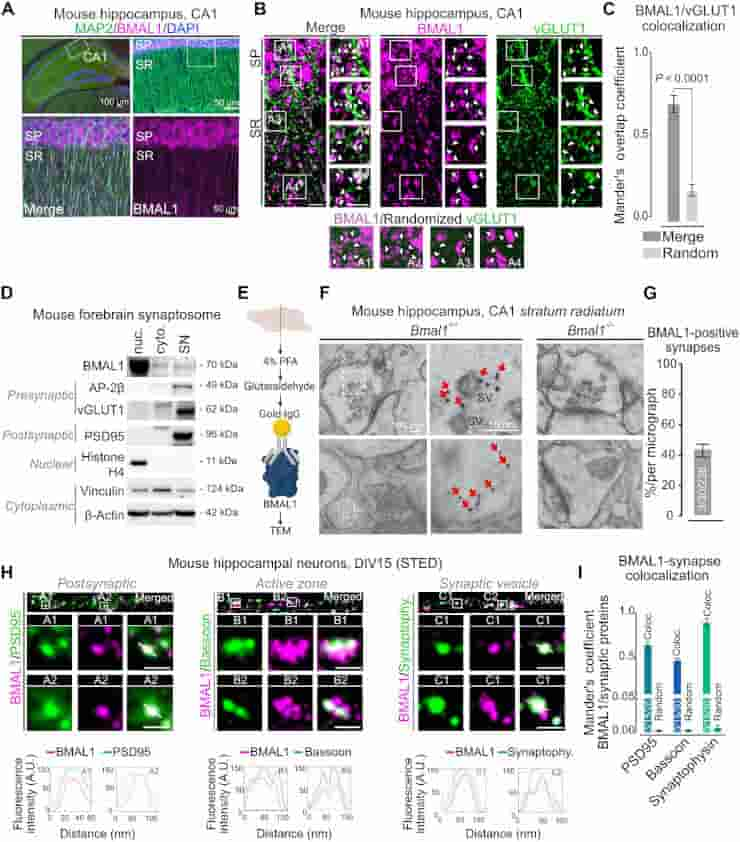
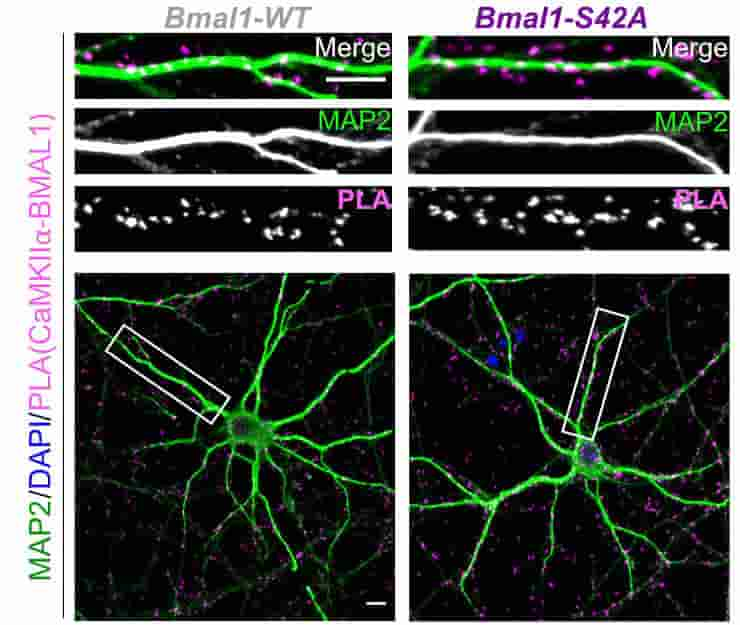
The researchers concentrated on the hippocampus, which exhibits significant circadian variations, and discovered that a tiny chemical change causes BMAL1 to interact with CaMKIIa, a major organizer of synaptic activity and memory formation. They were able to prevent such interaction without interfering with other key clock operations like as sleep/wake cycle timing or metabolism.
Beyond the hippocampus, Lipton and colleagues have also observed BMAL1 in synapses in the cortex and cerebellum, so their findings could extend to other brain functions.
Scientists can now begin to investigate how synaptic gating may be disrupted in neurodegenerative and neurodevelopmental diseases.
“We can look what’s happening to the rhythm of the clock protein and how can we optimize it. One exciting thing is that the interaction of BMAL1 and CaMKIIa is a biochemical event, phosphorylation, that can potentially be targeted with drugs. I feel like this is the beginning of lots of other work,”
Lipton said.
Abstract
The time of day strongly influences adaptive behaviors like long-term memory, but the correlating synaptic and molecular mechanisms remain unclear. The circadian clock comprises a canonical transcription-translation feedback loop (TTFL) strictly dependent on the BMAL1 transcription factor. We report that BMAL1 rhythmically localizes to hippocampal synapses in a manner dependent on its phosphorylation at Ser42 [pBMAL1(S42)]. pBMAL1(S42) regulates the autophosphorylation of synaptic CaMKIIα and circadian rhythms of CaMKIIα-dependent molecular interactions and LTP but not global rest/activity behavior. Therefore, our results suggest a model in which repurposing of the clock protein BMAL1 to synapses locally gates the circadian timing of plasticity.
References:
- Ilaria Barone et al. Synaptic BMAL1 phosphorylation controls circadian hippocampal plasticity. Sci. Adv. 9, eadj1010 (2023). DOI: 10.1126/sciadv.adj1010
- Lipton, Jonathan O et al. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell vol. 161,5 (2015): 1138-1151. doi:10.1016/j.cell.2015.04.002
Top Image: BMAL1 coimmunostaining of mouse CA1 stratum pyramidale and stratum radiatum. Credit: Sci. Adv. 9, eadj1010 (2023). DOI: 10.1126/sciadv.adj1010