Researchers at Tohoku University have shown that neuron–glial interactions in the cerebellum set the tone of aggression in a recent mouse study. This finding raises the possibility that future therapeutic approaches to control anger and aggression may depend on modulating glial activity in the cerebellum.
Aggression is frequently related to negative emotions. Aggression that is unchecked can lead to conflict, violence, and severe effects for individuals and society.
However, this does not mean that hostility is without purpose. It is an instinctual habit present in many species that may be important for survival. The idea is to manage and channel aggressiveness.
Bergmann Glial Cells
Scientists have lately recognized the cerebellum’s importance in non-motor tasks such as social cognition. In autism spectrum disorders and schizophrenia, a faulty cerebellum can contribute to social interaction issues.
It has been found that a region of the cerebellum known as the vermis is connected with aggression in humans. So the researchers studied the idea that Bergmann glial cells in the cerebellar vermis regulate the range of aggression in mice.
“Cells in the brain can be divided into neurons and glia, and although glia occupy approximately half of the brain, their participation in the brain’s information processing, plasticity, and health has long been an enigma. Our newly created fiber photometry method provides a gateway for understanding the physiology of glia,”
said study leader Professor Ko Matsui of the Super-network Brain Physiology lab at Tohoku University.
Resident and Intruder Combat
Matsui and his colleagues used the resident-intruder model, in which one mouse (the intruder) enters another mouse’s area (the resident). When an unfamiliar male mouse enters the cage, a sequence of conflicts between the resident male mouse and the intruder is typical.
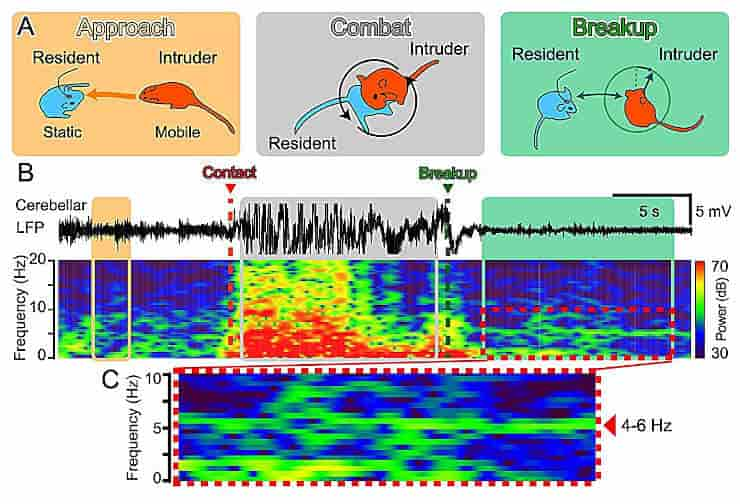
Each fight round lasted about 10 seconds, and these rounds were repeated at a frequency of approximately one per minute. The superiority and inferiority of the resident and intruder dynamically switched within each combat round.
The fiber photometry method revealed that intracellular Ca2+ levels in cerebellar glia decreased or increased in relation to the fight’s superiority or inferiority.
When the combat broke up, the researchers observed 4 to 6 Hz theta band local field potentials in the cerebellum, along with a sustained increase in Ca2+ levels in the glia. Optogenetic stimulation of cerebellar glia induced the emergence of the theta band, causing an early breakup of the fighting.
A Peaceful Future
Glia have been shown to control the local ionic and metabotropic environment in the brain and also to release transmitters that can affect neuronal activity in the vicinity. The findings of this study suggest that theta band cerebellar neuronal activity is regulated by the activity of Bergmann glial cells, demonstrating that cerebellar glial cells play a role in regulating aggression in mice.
Yuki Asano, the study’s lead investigator, believes that developing a therapeutic strategy that adjusts glial activity in the cerebellum could lead to future anger management strategies and clinical control of excessive aggression and violent behavior.
“Imagine a world without social conflict. By harnessing the innate ability of the cerebellar glia to control aggression, a peaceful future could be become reality,”
Asano said.
Reference:
- Yuki Asano et al. Glial tone of aggression. Neuroscience Research (2023). DOI: 10.1016/j.neures.2023.11.008
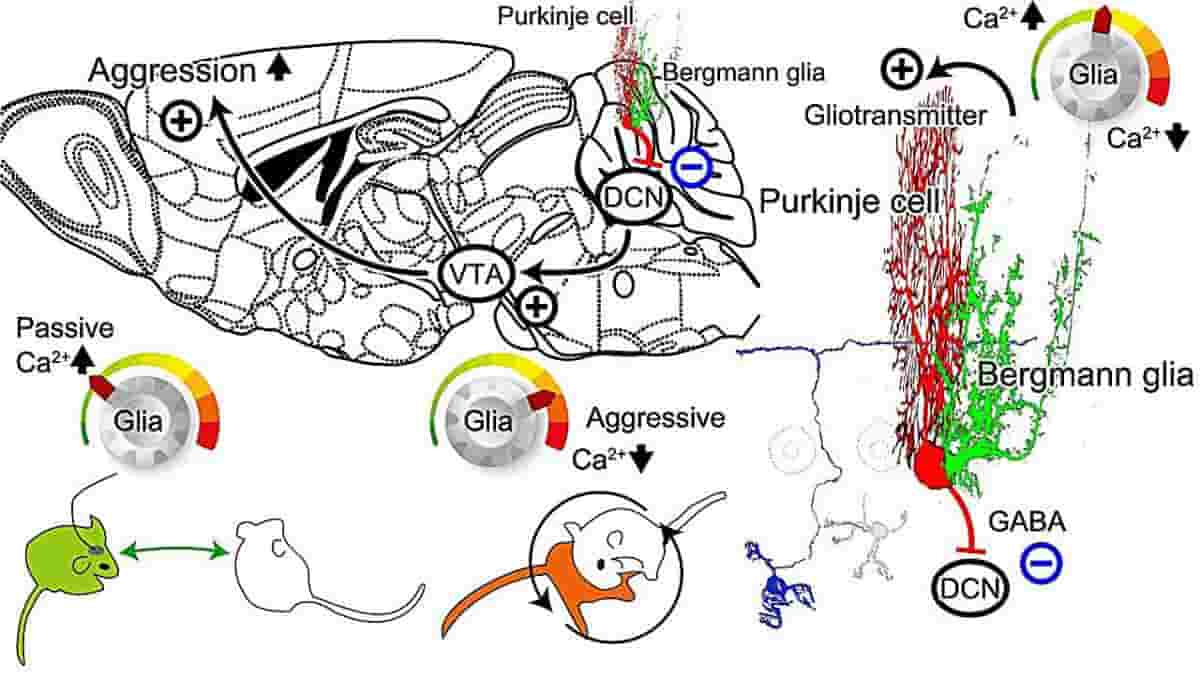
Top Image: Schematic diagram of the mechanism of cerebellar glial cell activity in regulating aggression. Gliotransmitter could be released in response to intracellular Ca2+ concentration increase in the cerebellar Bergmann glial cells. Excitatory transmitters such as glutamate would increase the cerebellar Purkinje cell activity. The only output nerve fiber from the cerebellum is the axon of the Purkinje cell (PC). PC activity leads to inhibitory GABA input to the deep cerebellar nuclei (DCN). Excitatory neuronal contacts from the DCN to the ventral tegmental area (VTA) have recently been identified. Dopaminergic neurons in the VTA have been reported to have a significant influence on social behavior, including aggression. Therefore, if the schematic pathway as described works, then an increase in Ca2+ concentration in cerebellar glial cells would be expected to increase PC activity and suppress DCN and VTA activity, leading to reduced aggression. This could lead to an early breakup of fights. On the other hand, a decrease in Ca2+ concentration in cerebellar glial cells would decrease PC activity and increase DCN and VTA activity, which would increase aggression and lead to dominance in fight situations. Credit: Yuki Asano, Ko Matsui