You’ll wait a little longer before drinking from a steaming teacup. And if your fingers get caught in the door, you’ll be more cautious the following time.
These are forms of associative learning, where a positive or negative experience leads to learning behavior. We know that our cerebellum is important in this form of learning. But how exactly does this work?
For a long time, it was believed that the cerebellum’s cortex, also referred to as the “little brain,” was in charge of associative learning. However, recent research from a collaboration between the Netherlands Institute for Neuroscience, Erasmus MC, and the Champalimaud Center for the Unknown suggests that the cerebellar nuclei play an unexpected role in this learning process.
Delay Eyeblink Conditioning
To look into this matter, a group of researchers from the Netherlands and Portugal, including Megan Carey, Cathrin Canto, and Chris de Zeeuw as senior authors, worked under the direction of Robin Broersen, Catarina Albergaria, and Daniela Carulli.
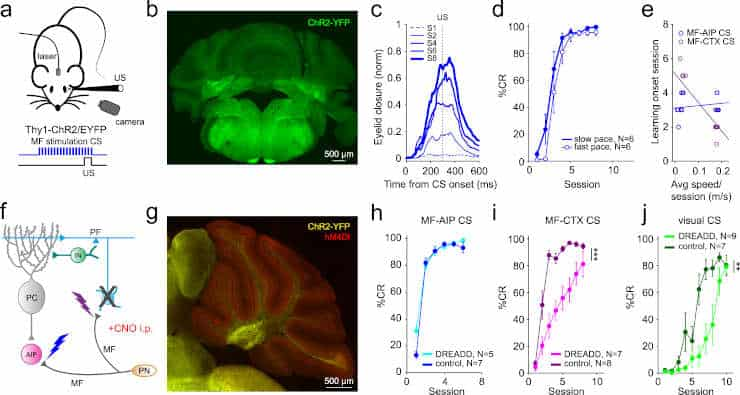
The researchers used two separate stimuli to teach the mice: a quick flash of light followed by a soft blast of air to the eye. The mice gradually learnt that there was a link between the two, leading them to close their eyes when they saw the flash of light. For many years, this behavioral paradigm, called delay eyeblink conditioning, has been employed to investigate how the cerebellum works.
“The cerebellar cortex has long been regarded as the primary player in learning the reflex and timing of the eyelid closure. With this study, we show that well-timed eyelid closures can also be regulated by the cerebellar nuclei. Both laboratories were working on similar research topics and when we realized the synergy of our work, we decided to start an international collaboration resulting in the present article,”
Robin Broersen said.
The Cerebellar Nuclei
If you look at the cerebellum, you can distinguish two major parts in it: the cerebellar cortex, or the outer layer of the cerebellum, and the cerebellar nuclei, the inner part. These parts are interconnected.
The nuclei are clusters of brain cells that receive information from the cortex. These nuclei, in turn, are linked to other brain locations that control motions such as eyelid closure. The nuclei are essentially the cerebellum’s output core.
The cerebellum is influenced by other brain regions via different connections, the so-called mossy fibers and the climbing fibers.
The mossy fibers in the experiment mentioned above are assumed to transmit information from the light, while the climbing fibers provide information relating to the air puff. This information then converges in the brain and cerebellar nuclei.
The Dutch team investigated the effect of associative learning on these connections to the nuclei and found that the mossy fibers had made stronger connections to the nuclei in the mice showing associative learning.
Well-timed Learning
Credit: Nat Commun 14, 7459 (2023). doi: 10.1038/s41467-023-43227-w
Meanwhile, the Portuguese team used optogenetics — a method that uses light to control cells — to test the capacity for learning in the cerebellar nuclei.
“Instead of using a regular light flash to train mice, we directly stimulated brain connections with light while pairing it with an air puff to the eye. This caused the mice to close their eyelids at the right times, showing that the cerebellar nuclei can support well-timed learning. To ensure this learning was actually happening in the nuclei, we repeated the experiments in mice with an inactivated cerebellar cortex,”
Catarina Albergaria said.
“While learning, connections between brain cells change. Still, it wasn’t clear where in the cerebellum these changes were taking place. Therefore, we looked at what happens to the mossy fibers and connections from the cortex while learning. We found that in mice that learned, but not ones that didn’t, the connections from the mossy fibers and from the cortex to the nuclei became stronger,”
Cathrin Canto added.
Huge Technical Challenge
The team also visualized what happens inside the cell by taking electrical measurements inside the nuclear cells of a living mouse.
“You can imagine that these cells are very small, 10 to 20 µm. That’s smaller than the diameter of a human hair. Using an ultra-thin tube with an electrode, we were able to record the electrical activity inside the cells while the mouse performed the task, an enormous technical challenge,”
Canto explained.
“In trained animals, light exposure caused the electrical activity inside the nucleus cells to change: the cells became more active the closer you got to the air puff in terms of timing,”
She said.
Learning Process Clarified
In essence, the cells could regulate the eyelid before the puff occurred because they were primed for what was about to happen and could make their electrical activity precise.
Although this study was conducted on mice, the general structure of the cerebellum is comparable in mice and humans. Despite the fact that humans have far more cells, the researchers expect the connections between cells to be arranged similarly.
“Our results contribute to a better understanding of how the cerebellum works and what happens during the learning process. This also leads to more knowledge about how damage to the cerebellum affects functioning, which may help patients in the future. By stimulating the connections to the nuclei using deep brain stimulation, it might be possible to learn new motor skills,”
Broersen said.
Abstract
Associative learning during delay eyeblink conditioning (EBC) depends on an intact cerebellum. However, the relative contribution of changes in the cerebellar nuclei to learning remains a subject of ongoing debate. In particular, little is known about the changes in synaptic inputs to cerebellar nuclei neurons that take place during EBC and how they shape the membrane potential of these neurons. Here, we probed the ability of these inputs to support associative learning in mice, and investigated structural and cell-physiological changes within the cerebellar nuclei during learning. We find that optogenetic stimulation of mossy fiber afferents to the anterior interposed nucleus (AIP) can substitute for a conditioned stimulus and is sufficient to elicit conditioned responses (CRs) that are adaptively well-timed. Further, EBC induces structural changes in mossy fiber and inhibitory inputs, but not in climbing fiber inputs, and it leads to changes in subthreshold processing of AIP neurons that correlate with conditioned eyelid movements. The changes in synaptic and spiking activity that precede the CRs allow for a decoder to distinguish trials with a CR. Our data reveal how structural and physiological modifications of synaptic inputs to cerebellar nuclei neurons can facilitate learning.
Reference:
- Broersen, R., Albergaria, C., Carulli, D. et al. Synaptic mechanisms for associative learning in the cerebellar nuclei. Nat Commun 14, 7459 (2023). doi: 10.1038/s41467-023-43227-w