Neuronal APOE4 is essential for the development of Alzheimer’s disease’s major pathologies, new research indicates.
The strongest genetic risk factor for developing late-onset Alzheimer’s disease is a gene for a protein called ApoE4. On average, people with one copy of this gene are 3.5 times more likely to develop Alzheimer’s disease than those without it, and those with two copies are 12 times more likely. It is still unknown how ApoE4 increases the risk of Alzheimer’s, though.
ApoE4 is produced by a variety of brain cell types, including neurons and glia, though glia produce more of it overall. Because of this, ApoE4 from glia has been the focus of the majority of earlier studies on this protein.
Now, researchers at the Gladstone Institutes are providing new perspectives on the neuronal production of ApoE4. ApoE4 from neurons plays a much larger disease-driving role in Alzheimer’s disease than previously believed, according to this new study conducted on mice.
“This research could be a turning point in the field of ApoE research. Our findings suggest an opportunity to explore new treatments that would specifically target neuronal ApoE4 to protect against Alzheimer’s disease,”
said senior author Yadong Huang, MD, Ph.D.
Astrocytic vs Neuronal ApoE4
ApoE4 is one of three forms of the ApoE protein, which is present in every individual’s body. The majority of individuals have the “healthy” variant, ApoE3, whereas approximately 12 percent have the Alzheimer’s-protective variant, ApoE2.
However, approximately 25% of North Americans have one copy of the ApoE4 gene, and up to 3% have two copies.
The majority of ApoE in our brains is produced by astrocytes, a type of glial cell, and previous research has suggested that astrocyte-derived ApoE4 contributes to Alzheimer’s disease. Nevertheless, evidence from Huang and colleagues has suggested for years that ApoE4 from neurons may play an even more significant role.
“Under normal conditions, neurons don’t make much ApoE at all, but when under stress or in response to injury, they quickly increase production of this protein. That’s why we are very interested in neuronal ApoE4 under disease conditions.”
said Huang, director of the Center for Translational Advancement at Gladstone, and professor of neurology and pathology at UC San Francisco (UCSF).
Tauopathy Mouse Model
To better understand the protein’s role, his team created an Alzheimer’s disease mouse model in which the mouse ApoE gene was replaced by the human ApoE4 or ApoE3 gene. The mice also have a variant of the human tau protein, which accumulates in the brain with age and is a hallmark of Alzheimer’s disease.
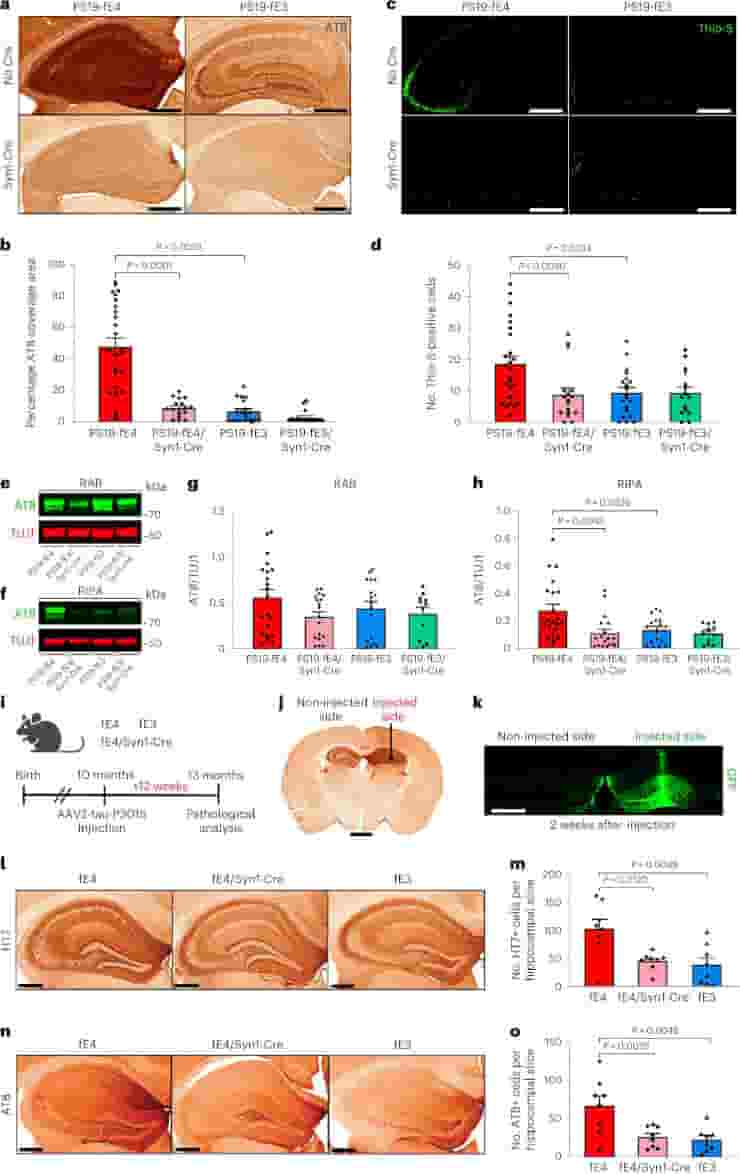
Importantly, in the genetically engineered mice, ApoE production by neurons could be selectively The scientists were able to investigate how deleting neuronal ApoE4 affects disease progression in mice that mimic Alzheimer’s disease by preserving ApoE production by other cell types.
The researchers discovered that removing ApoE4 from neurons reduced many brain changes similar to those in Alzheimer’s disease.
In the models, deleting neuronal ApoE4 reduced abnormal tau accumulation by more than 80%. It also protected against neuron loss and shrinkage of the hippocampus, a critical part of the brain for memory formation that degenerates in Alzheimer’s patients.
Extensive Reductions in Pathology
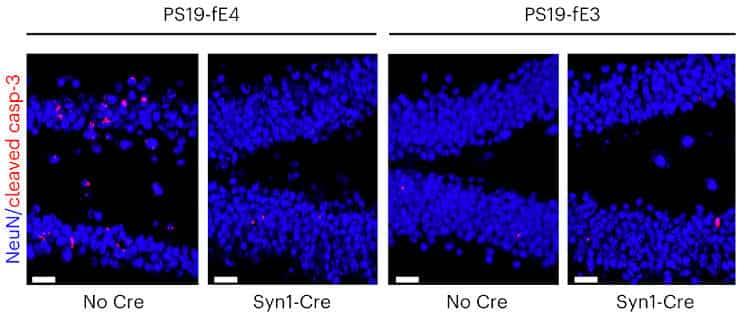
Furthermore, deleting neuronal ApoE4 protected against the loss of myelin sheaths. It reduced overexcitation of neurons, which is common in Alzheimer’s disease, as well as abnormally intense reactions of glial cells, which are key contributors to degeneration in the disease.
“These extensive reductions in pathology were striking, especially since neuronal ApoE4 only accounts for a small portion of the ApoE produced in the brain,”
says lead author Nicole Koutsodendris, Ph.D.
The findings strongly suggest that ApoE4 neuronal expression is required for the development of Alzheimer’s disease in ApoE4 carriers.
Treatment Development Potential
While some medications and other treatments may help slow the progression of Alzheimer’s disease or treat symptoms, no cure is currently available. And patients are in desperate need of help.
Huang’s team has created new avenues for the treatment of Alzheimer’s disease by demonstrating a critical function for neuronal ApoE4. For instance, drugs or gene-editing techniques could be created to block the processes that cause neurons to produce ApoE4.
“Our findings highlight the therapeutic potential of reducing neuronal ApoE4 in Alzheimer’s disease. They open up exciting new opportunities for the development of better drugs or strategies to treat this devastating disease,”
said Huang.
Reference:
- Koutsodendris, N., Blumenfeld, J., Agrawal, A. et al. Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat Aging (2023).
Last Updated on November 11, 2023