A groundbreaking approach developed by academics at the University of Oxford could one day provide personalized repairs for those who have suffered brain damage. For the first time, the researchers proved that neuronal cells may be 3D-printed to imitate the architecture of the cerebral cortex.
Brain injuries, including those caused by stroke, trauma, and brain tumor surgery, typically result in significant damage to the cerebral cortex (the outer layer of the human brain), resulting in difficulties with cognition, movement, and communication. For example, each year, around 70 million people globally suffer from traumatic brain injury (TBI), with 5 million of these cases being severe or fatal.
There are currently no effective treatments for severe brain injuries, resulting in serious impact on quality of life. Tissue regenerative therapies, particularly those in which patients get implants produced from their own stem cells, could be a viable future treatment option for brain injuries. However, there has been no way to assure that implanted stem cells mirror the architecture of the brain until now.
Structural and Functional Integration
The University of Oxford researchers created a two-layered brain tissue by 3D printing human neural stem cells in this new study. The cells displayed impressive structural and functional integration with the host tissue when implanted into mouse brain slices.
“This advance marks a significant step towards the fabrication of materials with the full structure and function of natural brain tissues. The work will provide a unique opportunity to explore the workings of the human cortex, and in the long term, it will offer hope to individuals who sustain brain injuries.
said lead author Dr. Yongcheng Jin, Department of Chemistry, University of Oxford.
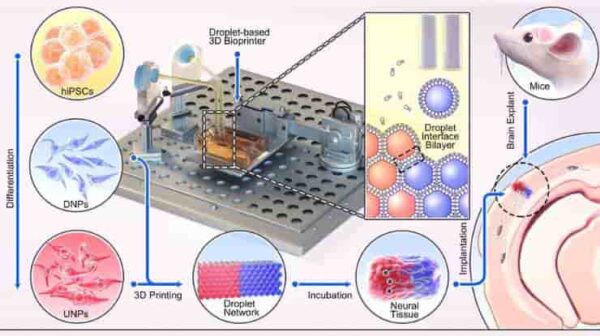
The cortical structure was made from human induced pluripotent stem cells (hiPSCs), which have the potential to produce the cell types found in most human tissues. A key advantage of using hiPSCs for tissue repair is that they can be easily derived from cells harvested from patients themselves and, therefore, would not trigger an immune response.
The 3D droplet printer is equipped with a piezo driver that generates mechanical pulses and expels aqueous droplets from a printing nozzle into oil. The ejected droplets, containing extracellular matrix only or extracellular matrix and cells, spontaneously acquire a lipid monolayer at the droplet/ oil interface, and contacting droplets form droplet-interface bilayers.
Using computer-aided printing, the cell-containing droplets – with a diameter of approximately 100 micrometres – can be arranged into various droplet network designs.
Communicative Signaling Activity
The hiPSCs were differentiated into neural progenitor cells for two different layers of the cerebral cortex by using specific combinations of growth factors and chemicals.
After that, the cells were suspended in solution to produce two “bioinks,” which were then printed to create a two-layered structure. The printed tissues retained their layered cellular architecture in culture for weeks, as evidenced by the expression of layer-specific biomarkers.
When the printed tissues were implanted into mouse brain slices, they showed strong integration, as demonstrated by the projection of neural processes and the migration of neurons across the implant-host boundary.
The implanted cells also showed signalling activity, which correlated with that of the host cells. This indicates that the human and mouse cells were communicating with each other, demonstrating functional as well as structural integration.
The researchers plan to improve the droplet printing technology in order to construct sophisticated multi-layered cerebral cortex tissues that more realistically replicate the architecture of the human brain. These created tissues may be employed in medication evaluation, brain development investigations, and to better our understanding of the basis of cognition, in addition to treating brain injuries.
Natural Bridge
The new advance builds on the team’s decade-long track record of inventing and patenting 3D printing technologies for synthetic tissues and cultured cells.
“Our droplet printing technique provides a means to engineer living 3D tissues with desired architectures, which brings us closer to the creation of personalized implantation treatments for brain injury,”
said senior author Dr. Linna Zhou.
“The use of living brain slices creates a powerful platform for interrogating the utility of 3D printing in brain repair. It is a natural bridge between studying 3D printed cortical column development in vitro and their integration into brains in animal models of injury,”
senior author Associate Professor Francis Szele, Department of Physiology, Anatomy and Genetics, University of Oxford, added.
The formation of the human brain is a delicate and intricate process with a complicated choreography. It is unrealistic to believe that we can duplicate the complete biological development in the lab.
Nonetheless, this 3D printing experiment shows significant progress in directing the fates and configurations of human iPSCs to build the cerebral cortex’s basic functional units, according to senior author Professor Zoltán Molnár.
Abstract
Engineering human tissue with diverse cell types and architectures remains challenging. The cerebral cortex, which has a layered cellular architecture composed of layer-specific neurons organised into vertical columns, delivers higher cognition through intricately wired neural circuits. However, current tissue engineering approaches cannot produce such structures. Here, we use a droplet printing technique to fabricate tissues comprising simplified cerebral cortical columns. Human induced pluripotent stem cells are differentiated into upper- and deep-layer neural progenitors, which are then printed to form cerebral cortical tissues with a two-layer organization. The tissues show layer-specific biomarker expression and develop a structurally integrated network of processes. Implantation of the printed cortical tissues into ex vivo mouse brain explants results in substantial structural implant-host integration across the tissue boundaries as demonstrated by the projection of processes and the migration of neurons, and leads to the appearance of correlated Ca2+ oscillations across the interface. The presented approach might be used for the evaluation of drugs and nutrients that promote tissue integration. Importantly, our methodology offers a technical reservoir for future personalized implantation treatments that use 3D tissues derived from a patient’s own induced pluripotent stem cells.
Reference:
- Jin, Y., Mikhailova, E., Lei, M. et al. Integration of 3D-printed cerebral cortical tissue into an ex vivo lesioned brain slice. Nat Commun 14, 5986 (2023). doi: 10.1038/s41467-023-41356-w