Neuropsychiatric symptoms that doctors commonly see in Alzheimer’s disease patients stem from brain inflammation rather than amyloid and tau proteins, University of Pittsburgh School of Medicine researchers report.
The discovery adds to the growing body of evidence supporting the role of neuroinflammation in Alzheimer’s disease progression and suggests new avenues for the development of therapies aimed at the disease’s neurological symptoms.
“Neuropsychiatric symptoms such as irritability, agitation, anxiety and depression are among the most difficult symptoms to treat in patients with Alzheimer’s. They are difficult to control, have no clear cause and make it difficult for families to care for their loved one without lots of support. Here, we show for the first time that brain inflammation may be to blame for these symptoms,”
said first author Cristiano Aguzzoli, M.D., postdoctoral associate at Pitt.
Microglial Activation
Earlier in 2023, Pitt researchers showed that increased brain inflammation is necessary for disease onset and can predict whether cognitively unimpaired elderly are more likely to acquire Alzheimer’s symptoms. Their previous research suggested that neuroinflammation was important in the pathologic cascade including other major actors in Alzheimer’s disease, such as amyloid beta and tau.
The new findings provide the first solid evidence that brain inflammation is a direct cause of neuropsychiatric symptoms that frequently accompany Alzheimer’s-related dementias.
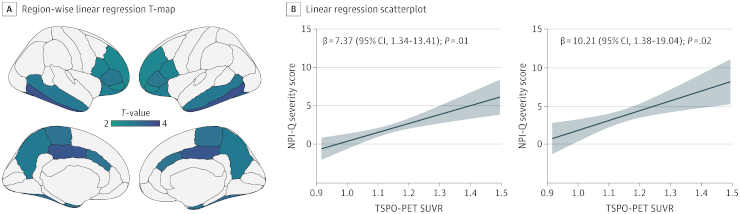
Credit: JAMA Netw Open. 2023; 6(11):e2345175. doi:10.1001/jamanetworkopen.2023.45175
By measuring levels of neuroinflammation, amyloid beta and tau via brain imaging and comparing the results with clinical assessments of neuropsychiatric symptom severity, the scientists discovered that microglial activation was strongly associated with a variety of neuropsychiatric symptoms, including disturbed sleep and agitation. While levels of amyloid and tau alone were predictive of neuropsychiatric symptoms, neuroinflammation seemed to have an added effect.
Targeting Neuroinflammation
Neuroinflammation was most closely linked to caretakers or family members describing their loved one’s rapid mood swings from calm to tears or fury, which is one of the disease’s main symptoms. Individuals whose caregivers showed higher levels of distress when caring for them had greater levels of brain inflammation.
Taken together, the findings contribute to the expanding body of evidence supporting the significance of brain inflammation in the early stages of illness progression, when symptoms such as excessive irritability appear. It also implies that clinical studies aimed at reducing neuroinflammation as a preventive medication for Alzheimer’s disease should track neuropsychiatric symptoms as one means of determining treatment efficacy.
On the other hand, medications that target neuroinflammation could potentially help lessen the severity of neuropsychiatric symptoms and lessen some of the psychological stress that caregivers experience, improving patient support.
“Since both neuroinflammation and neuropsychological abnormalities are found in several other types of dementia, including Parkinson’s dementia, we are collaborating with scientists around the world to expand these findings to these other diseases,”
said senior author Tharick Pascoal, M.D., Ph.D., associate professor of psychiatry and neurology at Pitt.
The work received support with grants from the Canadian Institutes of Health Research (CIHR); the Canadian Consortium of Neurodegeneration and Aging (CCNA); the Weston Brain Institute; and from the Alzheimer Association.
Abstract
Importance Neuropsychiatric symptoms are commonly encountered and are highly debilitating in patients with Alzheimer disease. Understanding their underpinnings has implications for identifying biomarkers and treatment for these symptoms.
Objective To evaluate whether glial markers are associated with neuropsychiatric symptoms in individuals across the Alzheimer disease continuum.
Design, Setting, and Participants This cross-sectional study was conducted from January to June 2023, leveraging data from the Translational Biomarkers in Aging and Dementia cohort at McGill University, Canada. Recruitment was based on referrals of individuals from the community or from outpatient clinics. Exclusion criteria included active substance abuse, major surgery, recent head trauma, safety contraindications for positron emission tomography (PET) or magnetic resonance imaging, being currently enrolled in other studies, and having inadequately treated systemic conditions.
Main Outcomes and Measures All individuals underwent assessment for neuropsychiatric symptoms (Neuropsychiatry Inventory Questionnaire [NPI-Q]), and imaging for microglial activation ([11C]PBR28 PET), amyloid-β ([18F]AZD4694 PET), and tau tangles ([18F]MK6240 PET).
Results Of the 109 participants, 72 (66%) were women and 37 (34%) were men; the median age was 71.8 years (range, 38.0-86.5 years). Overall, 70 had no cognitive impairment and 39 had cognitive impairment (25 mild; 14 Alzheimer disease dementia). Amyloid-β PET positivity was present in 21 cognitively unimpaired individuals (30%) and in 31 cognitively impaired individuals (79%). The NPI-Q severity score was associated with microglial activation in the frontal, temporal, and parietal cortices (β = 7.37; 95% CI, 1.34-13.41; P = .01). A leave-one-out approach revealed that irritability was the NPI-Q domain most closely associated with the presence of brain microglial activation (β = 6.86; 95% CI, 1.77-11.95; P = .008). Furthermore, we found that microglia-associated irritability was associated with study partner burden measured by NPI-Q distress score (β = 5.72; 95% CI, 0.33-11.10; P = .03).
Conclusions and Relevance In this cross-sectional study of 109 individuals across the AD continuum, microglial activation was associated with and a potential biomarker of neuropsychiatric symptoms in Alzheimer disease. Moreover, our findings suggest that the combination of amyloid-β– and microglia-targeted therapies could have an impact on relieving these symptoms.
Reference:
- Schaffer Aguzzoli C, Ferreira PCL, Povala G, et al. Neuropsychiatric Symptoms and Microglial Activation in Patients with Alzheimer Disease. JAMA Netw Open. 2023; 6(11):e2345175. doi:10.1001/jamanetworkopen.2023.45175