Mount Sinai researchers say they have employed AI, for the first time, to construct “HistoAge,” an algorithm that predicts age at death based on the cellular composition of human brain tissue specimens. It is said to have an average accuracy of 5.45 years.
This sophisticated method can also detect neuroanatomical regions prone to age-related alterations, which can be an indicator of prospective cognitive illnesses.
The aging brain undergoes structural and cellular changes that can impair function and increase susceptibility to neurodegenerative disorders such as Alzheimer’s.
Age acceleration — or the differences between biological and chronological age — in the brain can reveal insights about mechanisms and normal functions of one of the body’s most important organs. It can also explain age-related changes and functional decline, as well as identify early changes related to diseases, indicating the onset of a brain disorder.
Machine Learning Estimate
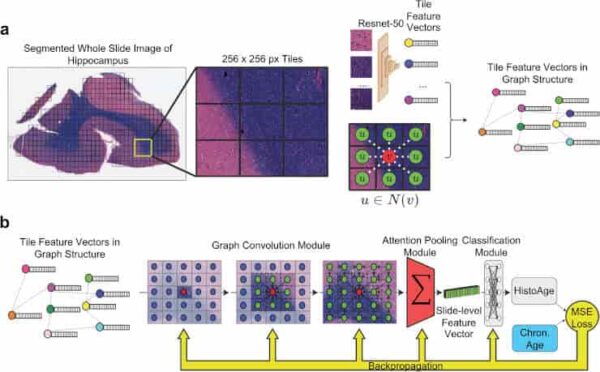
(a) Preprocessing: whole slide images (WSI) are segmented into 256 × 256 pixel tiles, removing areas without tissue. The tiles are then passed through an Imagenet pretrained Resnet-50 convolutional neural network, which yields feature vectors. Finally, the WSI is represented as a graph where each node is a tile encoded into a feature vector, with edges between tiles that are adjacent in slide space. (b) Model training: the WSI represented as a graph structure is passed through a graph convolution model, which learns not only the features of each tile but also messages passing between tiles, integrating macroscale information along with microscopic information. This is then passed through an attention pooling module, which pools all the tile feature vectors into a single slide-level feature vector. This is then passed through a classification model, which estimates HistoAge. HistoAge is taken with chronologic age and the mean-squared error (MSE) loss is used to update the weights of the model.
Credit: Acta Neuropathol (2023). doi:10.1007/s00401-023-02636-3 CC-BY
To construct the histological brain age assessment system, the researchers reviewed over 700 digitized pictures of slides of human hippocampal sections from elderly brain donors. Because the hippocampus is known to be implicated in both brain aging and age-related neurodegenerative disorders, it is a suitable location for investigation.
The scientists then trained a machine learning model to estimate a person’s age at death based on the digitized segment, a task that a human observer would be unable to execute with any degree of precision. They calculated the amount of age acceleration in the brain by subtracting the model-predicted age from the actual age.
When compared with current measures of age acceleration (e.g., DNA methylation), they found that HistoAge-based age acceleration had stronger associations with cognitive impairment, cerebrovascular disease, and the levels of Alzheimer’s-type abnormal degenerative protein aggregation. The study found that the HistoAge model is a reliable, independent metric for determining brain age and understanding factors that drive neurodegeneration over time.
Brain Research Paradigm Shift
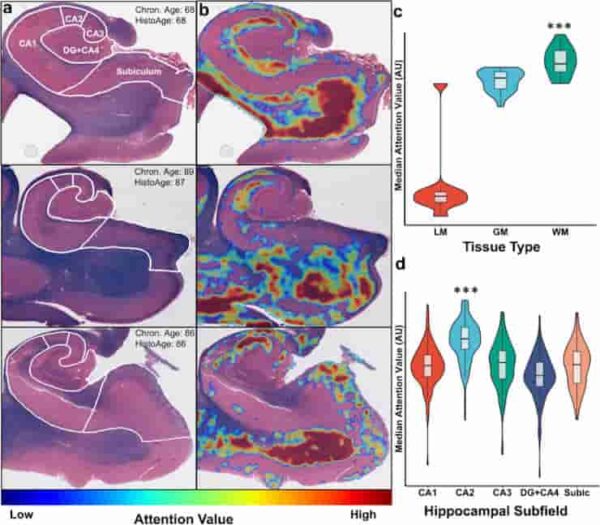
(a) Three representative Luxol-fast blue counterstained hematoxylin & eosin (H&E)-stained hippocampus sections with subfield labels, chronologic age, and estimated HistoAge are displayed. (b) Corresponding attention-weighted image, where high attention values are shown in red and low attention values are shown in blue. (c) Distribution of attention levels by tissue type. WM had (p < 0.001) higher attention than both GM and LM and GM had significantly (p < 0.001) higher attention than LM. (d) Distribution of median attention values by hippocampal subfield, with the median attention value for each subfield displayed. One-way ANOVA of the distributions had an F = 78.9, p < 0.001. Subsequent Tukey’s test revealed CA2 to have significantly (p < 0.001) higher attention than all other subfields. DG dentate gyrus; Subic subiculum; LM leptomeninges; GM gray matter; WM white matter.
Credit: Acta Neuropathol (2023). doi:10.1007/s00401-023-02636-3 CC-BY
The HistoAge model, and subsequent comparable algorithms, provide an altogether new paradigm for measuring aging and neurodegeneration in human samples, according to the researchers, and may be easily applied at scale in clinical and translational research facilities. Furthermore, this method gives more rigorous, unbiased, and robust measures of the molecular alterations that underpin degenerative illnesses.
The team will next conduct a multicenter collaboration to develop a large AI-ready dataset that will be used to develop even more powerful AI models that have the potential to transform and enhance our understanding of brain diseases.
“AI’s disruptive influence on brain research is a paradigm shift propelling us towards the next generation of cures. The HistoAge model will enable us to uncover crucial causal aspects of debilitating brain diseases such as Alzheimer’s disease,”
Mount Sinai’s Dr. John F. Crary said.
AI is being used more and more by clinical scientists in research and diagnosis contexts. It’s a tool that’s reshaping medicine – not to replace our existing health system’s dedication to compassionate care, but to improve diagnosis and treatment for all patients.
“This model opens the floodgates for a slew of fascinating and essential analyses that bring us closer to finally understanding the aging brain and age-related brain diseases such as Alzheimer’s. This is the first time we have been able to put a number to how much aging there is in the brain in pathology. With this approach, we can discover genes that protect against brain aging or genes that make aging worse in the brain, as well as discover the environmental risk factors that make individuals’ brains age faster,”
said Mount Sinai’s Gabriel Marx, a co-author of the paper.
Abstract
Understanding age acceleration, the discordance between biological and chronological age, in the brain can reveal mechanistic insights into normal physiology as well as elucidate pathological determinants of age-related functional decline and identify early disease changes in the context of Alzheimer’s and other disorders. Histopathological whole slide images provide a wealth of pathologic data on the cellular level that can be leveraged to build deep learning models to assess age acceleration. Here, we used a collection of digitized human post-mortem hippocampal sections to develop a histological brain age estimation model. Our model predicted brain age within a mean absolute error of 5.45 ± 0.22 years, with attention weights corresponding to neuroanatomical regions vulnerable to age-related changes. We found that histopathologic brain age acceleration had significant associations with clinical and pathologic outcomes that were not found with epigenetic based measures. Our results indicate that histopathologic brain age is a powerful, independent metric for understanding factors that contribute to brain aging.
Reference:
- Marx, G.A., Kauffman, J., McKenzie, A.T. et al. Histopathologic brain age estimation via multiple instance learning. Acta Neuropathol (2023). doi:10.1007/s00401-023-02636-3