According to a mouse study, a newly discovered brain connection can explain how early-life stress and adversity disrupt the operation of the brain’s reward circuit.
This circuit’s dysfunction is thought to be at the root of several major disorders, including depression, substance abuse, and excessive risk-taking. The discovery provides a new therapeutic target for treating mental illnesses.
“We know that early-life stress impacts the brain, but until now, we didn’t know how,”
said senior author Tallie Z. Baram, of the University of California, Irvine.
Stress and Brain Development
Stress can affect the developing brain in various ways. A 2019 study found that white matter in the brain was altered in babies of mothers who experienced greater stress during pregnancy and the period before birth.
Infants’ early exposure to pain alters neural circuits in the brain that regulate stress. And the stress of the COVID pandemic changed the brains of teenagers in a way that made their brain structures look several years older than they should have.
In this study, the researchers were looking for potentially stress-sensitive brain pathways. They discovered a new pathway within the reward circuit that expresses a corticotropin-releasing hormone (CRH) molecule, which regulates our stress responses. They discovered that negative experiences cause this brain pathway to become overactive.
“These changes to the pathway disrupt reward behaviours, reducing pleasure and motivation for fun, food, and sex cues in mice. In humans, such behavioural changes, called anhedonia, are associated with emotional disorders,”
Baram said.
Importantly, the researchers discovered that silencing this pathway with modern technology restores the normal reward behaviours of the brain.
Gamma-aminobutyric Acid
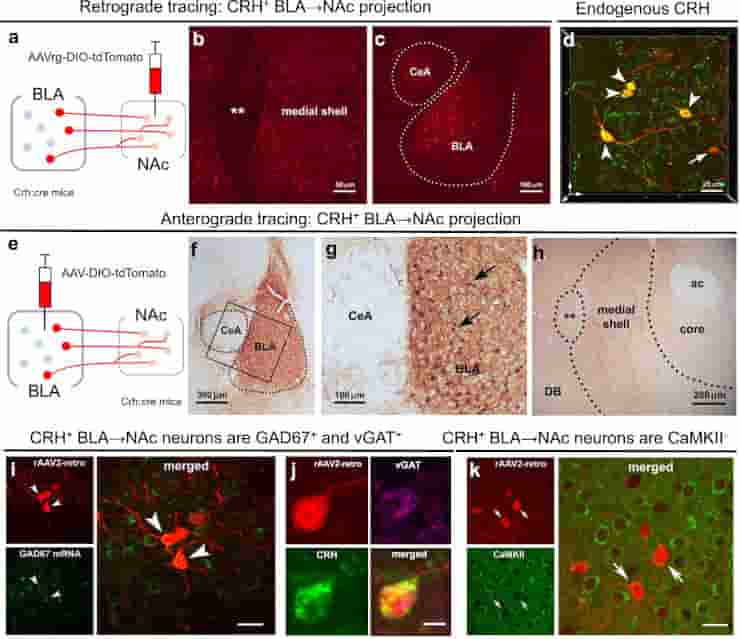
(d) 3D image (z-stack; 0.5 μm steps) confirmed localization in the BLA of AAV-retro infected cells (red) that co-express endogenous CRH (green); dual labeled neurons = yellow. (e–g) Anterograde tracing of CRH+ axonal projections from BLA to medial NAc shell. (e) The AAV1-DIO-tdTomato construct and the viral genetic experimental design. (f) Virus injection is confined to the BLA, (g) and absent from the central amygdala (CeA), shown by selective expression of tdTomato in BLA CRH+ neurons. h BLA-origin CRH+ axons and terminals in the medial NAc shell. (i–k) Virus injection into the medial NAc shell retrogradely infected somata in the BLA. (i) Combined fluorescence in situ hybridization (FISH) and immunostaining with GAD67 mRNA in CRH+ cells in the BLA. Arrowheads point to co-localized GAD67 mRNA and virus-reporter labeling. (j) a BLA → NAc cell (red) co-expresses endogenous CRH (green) and vGAT (magenta), but (k) does not co-express the glutamatergic marker CaMKII. ** = Major Island of Calleja, ac anterior commissure, DB diagonal band.
Credit: Nat Commun 14, 1088 (2023) CC-BY
Researchers mapped all of the CRH-expressing connections to the nucleus accumbens, the brain’s pleasure and motivation hub, and discovered a previously unknown projection originating from the basolateral amygdala. Along with orticotropin-releasing hormone, projection fibres also expressed gamma-aminobutyric acid.
When stimulated, this new pathway suppresses multiple types of reward behaviours in male mice.
The study included male and female mice in two separate groups. One was subjected to early adversity by spending a week in cages with limited bedding and nesting material, while the other was raised in standard cages.
Early Life Adversity
As adults, male mice exposed to early adversity had less interest in sweet foods and sex cues than typically raised mice. In contrast, females experiencing adversity craved sweet, fatty foods. The inhibition of the pathway restored normal reward behaviour in males but had no effect on females.
“We believe that our findings provide breakthrough insights into the impact of early-life adversity on brain development and specifically on control of reward behaviors that underlie many emotional disorders,”
Baram said.
The team’s discovery of the previously unidentified circuit function of the basolateral amygdala-nucleus accumbens brain pathway deepens our understanding of this complex mechanism and identifies an important new therapeutic target. Future research will be required to increase our knowledge of early-life adversity’s distinct and gender-specific behavioural effects.
Reference:
- Birnie, M.T., Short, A.K., de Carvalho, G.B. et al. Stress-induced plasticity of a CRH/GABA projection disrupts reward behaviors in mice. Nat Commun 14, 1088 (2023)
Last Updated on December 13, 2023
