Blueprints that direct human cells to assemble a virus-like delivery system for transporting custom cargo from one cell to another have been developed by scientists from the University of Utah and University of Washington.
The work is a step toward a nature-inspired means for delivering therapeutics directly to specific cell types within the body. Wesley Sundquist, Ph.D., co-chair of the Department of Biochemistry at the University of Utah School of Medicine, said:
“We’re shifting our perception from viruses as pathogens, to viruses as inspiration for new tools,”
Sundquist is co-senior author on the study, along with Neil King, Ph.D., assistant professor at the Institute for Protein Design at the University of Washington.
Blueprint For Nanocages
The carefully designed instructions set forth a series of self-propelled events that mimic how some viruses transfer their infectious contents from one cell to the next.
From the blueprints tumbled out self-assembling, soccer ball-shaped “nanocages”. Adding on specific pieces of genetic code from viruses caused the nanocages to be packaged within cell membranes, and then exported from cells.
Like a shuttle leaving Earth to bring goods to a space station, the tiny capsules undocked from one cell, traveled to another and docked there, emptying its contents upon arrival.
In this experiment, the protective nanocages carried cargo that the scientists used like homing beacons to track the vessels’ journeys.
Next steps are to design nanocages that hold drugs or other small molecules that would be assembled factory-style in one set of cells, and sent out from there. Such biologically-based delivery systems are expected to be better tolerated by the body than other nanoparticles made from synthetic materials.
“We are now able to accurately and consistently design new proteins with tailor-made structures,” says King. “Given the remarkably sophisticated and varied functions that natural proteins perform, it’s exciting to consider the possibilities that are open to us.”
Viral Efficiency
The researchers’ decision to model the microscopic shipping system after viruses was no accident. Viruses have honed their skills to spread their infectious wares to large numbers of cells effectively.
Decades of research, including in-depth investigations of the human immunodeficiency virus (HIV) by Sundquist’s team, have led to an understanding of how the pathogens accomplish this goal with such efficiency.
One test of whether you truly understand something is to build it yourself. And that’s what Sundquist and King’s teams have done here.
“The success of our system is the first formal proof that this is how virus budding works,” remarks Sundquist.
Viruses taught them that such a delivery system must include three essential properties: an ability to grasp membranes, self-assemble, and to be released from cells. Introducing coding errors into any one of those steps brought shipments to a halt.
“I was sure that this would need fine-tuning but it was clean from the very beginning,”
commented lead author Jörg Votteler, Ph.D., a postdoctoral fellow in biochemistry at the University of Utah.
The system could be modified as long as the three basic tenets were left intact. For example, the scientists could swap in differently shaped cages, or cause another type of membranes to surround them.
Modularity means the vessels can be customized for various applications.
This study is proof of principle that the systems works, but more needs to be done before it can be applied therapeutically. Researchers will need to determine whether the capsules can navigate long journeys within living animals, for instance, and whether they can deliver medicines in sufficient quantities.
Reference:
- Jörg Votteler et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature (2016). DOI: 10.1038/nature20607
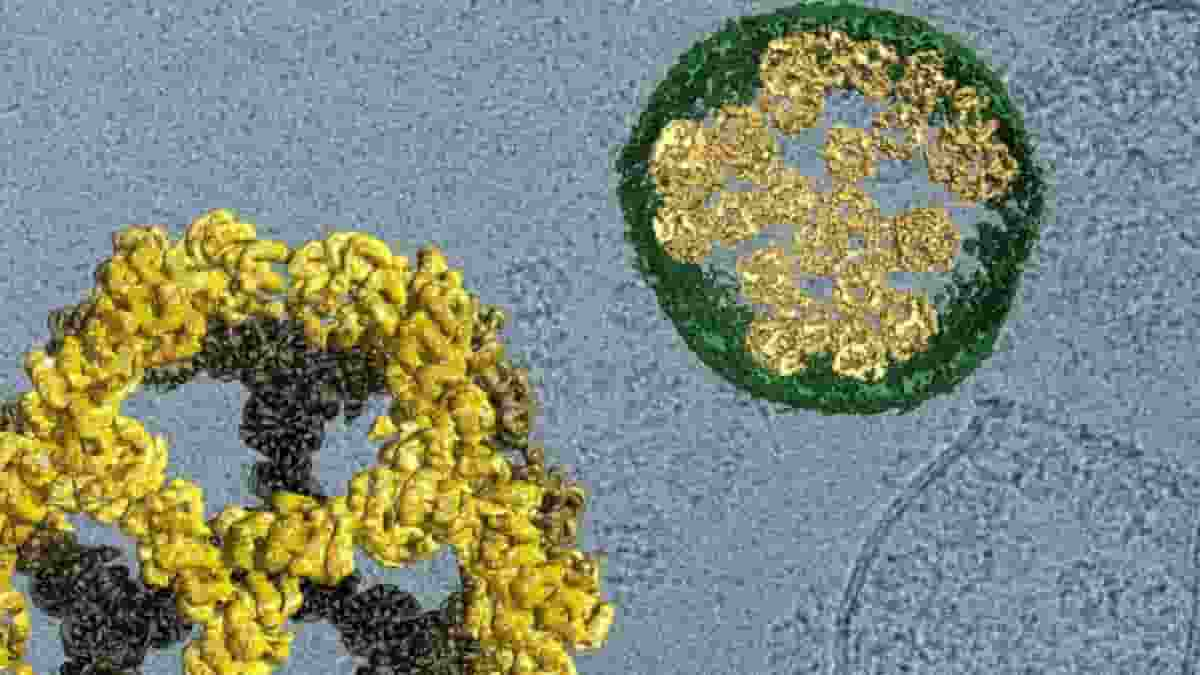
Image: Cargo is encased in protein nanocages (yellow, enlarged on left) that are carried from one cell to another within vesicles built from membranes (green, shown in cross-section). Credit: David Belnap, Jörg Votteler
Last Updated on October 30, 2023