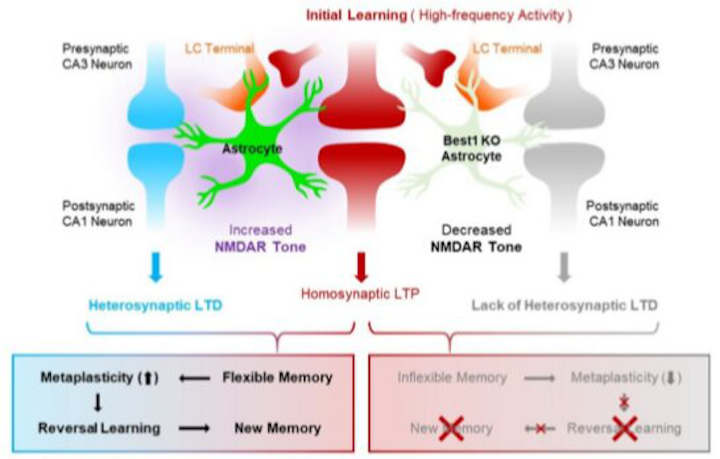
The ability of astrocytes to simultaneously regulate and integrate synaptic plasticity of nearby synapses is important for facilitating cognitive flexibility, researchers in South Korea report.
It is thought that lower cognitive flexibility in brain disorders such as autism, schizophrenia, and early stages of Alzheimer’s disease is caused by the reduced function of N-methyl-D-aspartate receptors (NMDARs). While NMDARs are important receptors for synaptic plasticity and are activated by a number of agonists and co-agonists, the source of one of the co-agonists, D-serine, has been controversial1.
Using astrocyte-specific gene regulation, the researchers, led by C. Justin Lee, director of the Center for Cognition and Sociality within the Institute for Basic Science (IBS) in Daejeon, showed2 that astrocytes can actually synthesize D-serine and release it through the calcium-activated channel called Best1.
Combined with the previous knowledge3: that astrocytes can release glutamate through Best1, the co-release of D-serine and glutamate indicates that astrocytes are ideal regulators of NMDAR activity and synaptic plasticity.
Cognitive Flexibility
Our brains need to not only learn new things but also to modify existing memories. This is commonly referred to as cognitive flexibility.
Without this ability, we would not be able to adapt to the altered environment and be vulnerable to making wrong choices from relying only on past memories.
In this study, the researchers were able to demonstrate that heterosynaptic long-term depression (LTD), a phenomenon in which inactive synapses weaken when nearby synapses become active, is mediated by astrocytes and is critical for cognitive flexibility.
Since each astrocyte is in contact with over 100,000 synapses, astrocytes can control numerous synapses and integrate synaptic plasticity simultaneously,
said first author Koh Wuhyun.
Memory Modification Issues
The researchers investigated the Best1 knockout (Best1 KO) mouse model that lacks heterosynaptic LTD due to reduced NMDAR tone. In the Morris Water Maze experiment, which involves mice trying to find a hidden platform, Best1 KO mice performed similarly to the wild-type mice in the initial learning session.
However, when the platform was relocated to the opposite side, Best1 KO mice exhibited problems in memory modification.
Interestingly, when the NMDAR tone was improved in Best1 KO mice by D-serine injection during the initial learning period, their memory modification problem was restored in the subsequent experiment. This discovery shows that memory flexibility is determined from the time of initial learning, which is different from the previously proposed theory that synaptic plasticity only occurs when memory modification is needed.
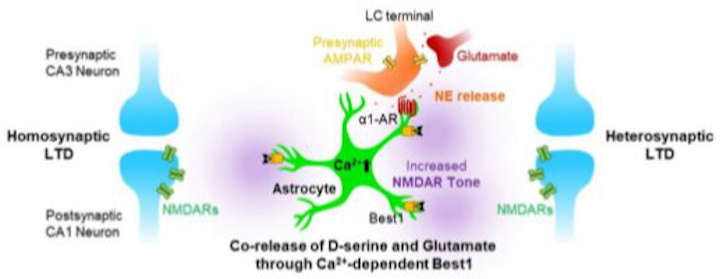
Additionally, the researchers discovered that norepinephrine and its receptor α1-AR can activate astrocytes and cause co-release of D-serine and glutamate. This implies that the flexibility of memory can be determined by the degree of concentration and arousal during the learning period.
Previous studies have mostly focused on changes in specific synapses to stimuli. The discovery of this phenomenon where changes in one synapse can induce changes in nearby synapses during learning shows that finding out what happens to the other synapses is important for understanding the mechanism of learning and memory formation. It is hoped that this study will provide valuable insights on how to relieve or treat symptoms of autism, schizophrenia, and early dementia, which are known to reduce cognitive flexibility,
C. Justin Lee said.
- Wuhyun Koh et al. Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus. Biological Psychiatry (2021). DOI: 10.1016/j.biopsych.2021.10.012 ↩︎
- Papouin T., Henneberger C., Rusakov D.A.
Oliet S.H.R. Astroglial versus neuronal D-serine: Fact checking. Trends Neurosci. 2017; 40: 517-520 ↩︎ - Han KS, Woo J, Park H, Yoon BJ, Choi S, Lee CJ. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol Brain. 2013, 6 (1): 4-10.1186/1756-6606-6-4. ↩︎
Last Updated on October 14, 2022