The human brain consumes nearly one-quarter of the body’s sugar energy, or glucose, daily. Gladstone Institutes and UC San Francisco researchers have now shed new light on how neurons consume and metabolize glucose and how these cells adapt to glucose shortages.
Previously, scientists suspected that much of the glucose used by the brain was metabolized by glia, which are brain cells that support the activity of neurons.
Previous research has shown that the brain’s uptake of glucose is reduced in the early stages of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. The new findings may lead to the development of new therapeutic approaches for those diseases, as well as a better understanding of how to keep the brain healthy as it ages.
“We already knew that the brain requires a lot of glucose, but it had been unclear how much neurons themselves rely on glucose and what methods they use to break the sugar down,”
said senior author Ken Nakamura, MD, Ph.D. Scientists now have a much better understanding of the fundamental fuel that drives neurons.
Neuronal Energy Metabolism Debate
Many of the foods we consume are broken down into glucose, which is then stored in the liver and muscles, transported throughout the body, and metabolized by cells to power the chemical reactions that keep us alive.
Scientists have long debated what happens to glucose in the brain, with many claiming that neurons do not metabolize the sugar. Instead, they proposed that glial cells consume the majority of the glucose and then indirectly fuel neurons by passing them a metabolic product of glucose called lactate.
However, evidence to support this theory has been limited, owing in part to how difficult it is for scientists to create cultures of neurons in the lab that do not also contain glial cells.
Nakamura’s team solved this problem by generating pure human neurons from induced pluripotent stem cells (iPS cells). Scientists can use IPS cell technology to transform adult cells from blood or skin samples into any cell type in the body.
Neurons Require Glucose Uptake
The generated neurons were mixed with a labelled form of glucose that the researchers could track even as it was broken down. This experiment demonstrated that neurons can absorb glucose and break it down into smaller metabolites.
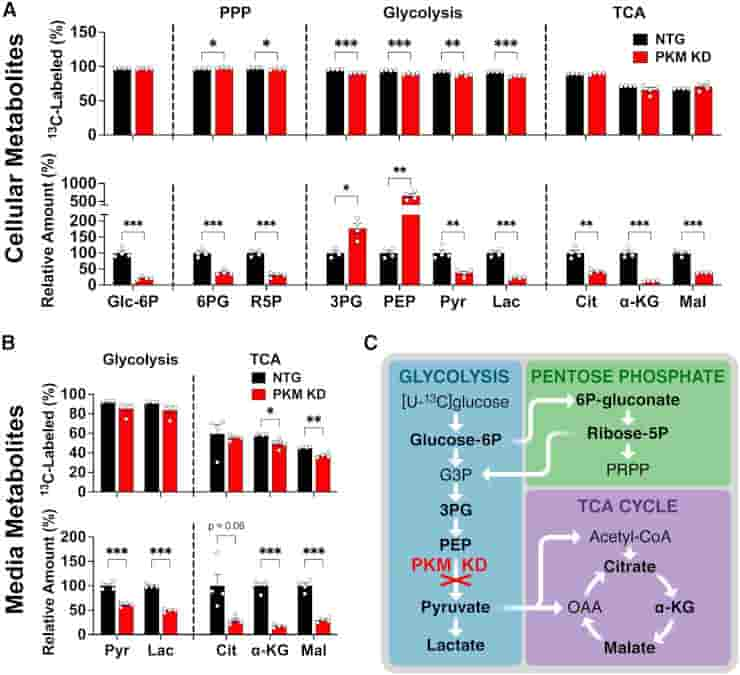
(C) Schematic of PKM KD impact on [U-13C]glucose metabolism.
Credit: Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112335
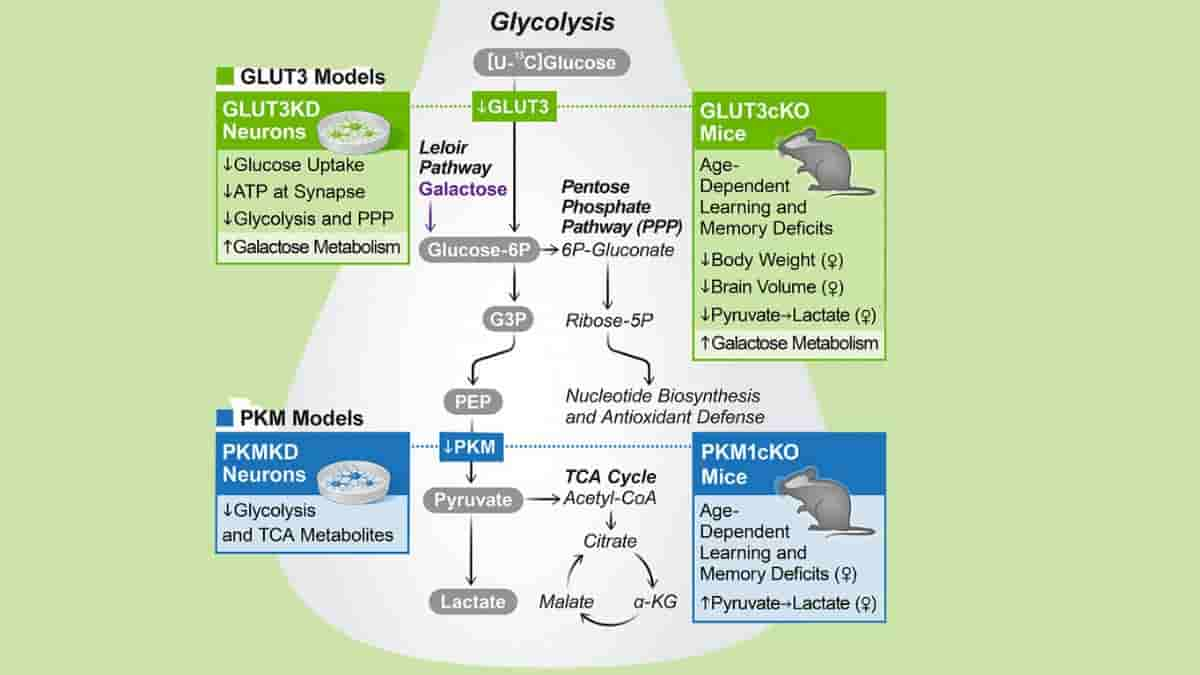
To determine how neurons used the products of metabolized glucose, the researchers used CRISPR gene editing to remove two key proteins from the cells.
One of the proteins facilitates glucose import into neurons, while the other is required for glycolysis, the primary pathway by which cells typically metabolize glucose. Taking either of these proteins out of the equation stopped the breakdown of glucose in isolated human neurons.
“This is the most direct and clearest evidence yet that neurons are metabolizing glucose through glycolysis and that they need this fuel to maintain normal energy levels,”
said Nakamura.
Sex-specific Requirement for Neuronal GLUT3
Nakamura’s team then turned to mice to investigate the role of neuronal glucose metabolism in living animals.
They engineered the neurons in the animals, but not the other types of brain cells, to lack the neuronal glucose transporter (GLUT3 and GLUT4) proteins required for glucose import and glycolysis. As a result, as the mice aged, they developed severe learning and memory problems.
![Female GLUT3cKO mice have smaller CA1 and total brain volumes, and decreased HP [1-13C]lactate-to-pyruvate ratios](https://sciencebeta.com/wp-content/uploads/2023/04/neuronal-glycolosis3-962x1024.jpg)
(B) Total brain and thalamus volumes calculated from in vivo T2-weighted images of 10- to 14-month-old mice were smaller in female GLUT3cKO mice, while ventricle volume was unchanged. n = 6–7 mice/group.
(C) Representative ex vivo T2-weighted images of 19-month-old mice used for volumetric analyses. CA1, hippocampal, and entire brain volumes were smaller in female GLUT3cKO mice. n = 3 mice/group.
(D and E) [18F]FDG-PET signal from the hippocampus (D) and specifically from the CA1 area (E) was similar between 12- and 14-month-old females and males. n = 6 mice/group.
(F) Representative 13C spectra of 8- to 14-month-old mice showing HP [1-13C]pyruvate and HP [1-13C]lactate levels from a region containing CA1 (red square) for female and male mice. HP [1-13C]lactate-to-pyruvate ratios were significantly lower in female GLUT3cKO mice. n = 6–7 mice/group.
Credit: Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112335
According to Nakamura, this indicates that neurons are not only capable of metabolizing glucose, but also rely on glycolysis for normal functioning.
“Interestingly, some of the deficits we saw in mice with impaired glycolysis varied between males and females. More research is needed to understand exactly why that is,”
he added.
Hyperpolarized Carbon-13
Myriam M. Chaumeil, Ph.D., associate professor at UCSF and co-corresponding author of the new study, has been developing specialized neuroimaging approaches based on a new technology called hyperpolarized carbon-13 that reveal the levels of specific molecular products. Her group’s imaging revealed how the mice’s brains’ metabolism changed when neurons blocked glycolysis
“Such neuroimaging methods provide unprecedented information on brain metabolism. The promise of metabolic imaging to inform fundamental biology and improve clinical care is immense; a lot remains to be explored,”
said Chaumeil.
The imaging findings helped prove that living animals’ neurons metabolize glucose via glycolysis. They also demonstrated the utility of Chaumeil’s imaging method for studying how glucose metabolism changes in people with diseases such as Alzheimer’s and Parkinson’s.
Alternative Energy Sources
Nakamura and his colleagues then investigated how neurons adapt when they are unable to obtain energy via glycolysis, as may be the case in certain brain diseases.
It was discovered that neurons use alternative energy sources, such as the related sugar molecule galactose. However, the researchers discovered that galactose was not as efficient as glucose as a source of energy and could not fully compensate for the loss of glucose metabolism.
In collaboration with Chaumeil’s team, his lab is planning future studies on how neuronal glucose metabolism changes with neurodegenerative diseases and how energy-based therapies could target the brain to improve neuronal function.
Limitations
The study has a few limitations that should be mentioned. The pure neuronal population studied lacks glial cells, circuits, and three-dimensional interactions found in vivo, all of which are thought to influence brain metabolism.
As a result, the relative flux of glucose metabolites via glycolysis versus the pentose phosphate pathway and downstream glycolytic pathways may differ between the human neurons studied and human and mouse neurons in vivo.
Despite the fact that this paper focuses on changes in neurons, the in vivo hyperpolarized pyruvate and MRI studies lack cell-type resolution. The team has begun to address this by specifically deleting GLUT3 and neuronal-enriched pyruvate kinase isoform (PKM1) in neurons postnatally.
These findings indicate that neurons require glycolysis in order to maintain normal brain energy metabolism and volume. They do not, however, prove conclusively that changes in either hyperpolarized pyruvate metabolism or brain volume are caused by changes in neurons rather than by non-cell-autonomous effects on glia.
Furthermore, the requirement for neuronal glycolysis does not reveal whether neurons also require astrocyte derived lactate and, if so, the energy balance derived from neuronal glucose metabolism versus astrocyte-derived lactate.
Reference:
- Li H, Guglielmetti C, Sei YJ, et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023;42(4):112335. doi:10.1016/j.celrep.2023.112335
Last Updated on September 19, 2023