As nerve cells develop, they build brain connections that allow for communication between them. The axon, one of these wires, lengthens and forms the foundation of neuronal networks. At the same time, nerve cells move to the cortex region.
Surprisingly, each of these dynamic processes is controlled independently, so the axon can keep growing even after the nerve cell has reached its final position and is ready to connect with its target cells.
“We found that the centrosome — an organelle that drives cell division — regulates the nerve cell migration; for the formation and growth of the axon, however, it does not play a role,”
first authors Dr. Stanislav Vinopal and Dr. Sebastian Dupraz of the German Center for Neurodegenerative Diseases (DZNE) said.
Centrosome Puzzle
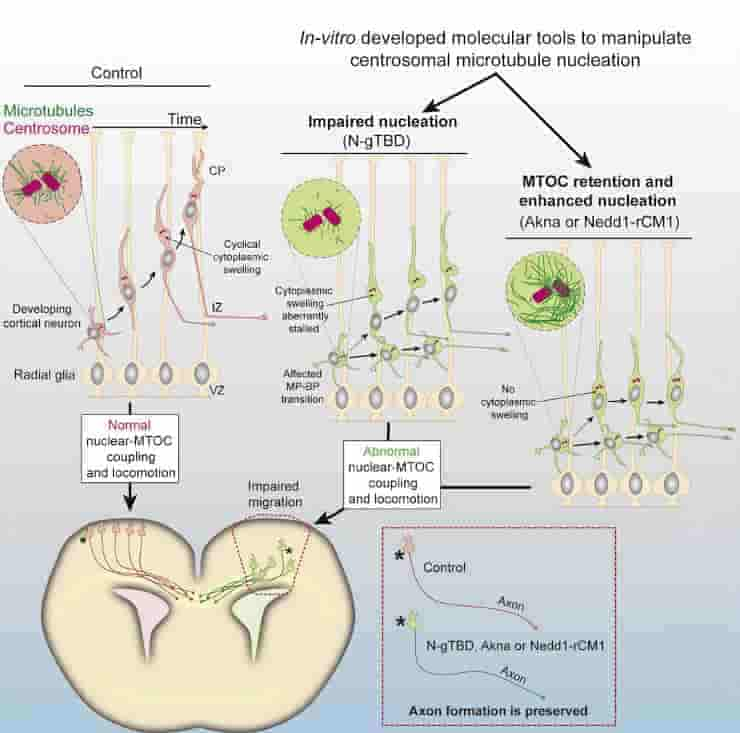
The function of the centrosome has been a point of contention until now. A dynamic skeleton found in cells called the cytoskeleton promotes growth and migration.
The cytoskeleton is made up of microscopic tubules known as microtubules. They also serve as the axon’s backbone. The centrosome is capable of producing microtubules.
With their findings, the researchers from Professor Dr. Frank Bradke’s group have solved a central puzzle in the field of neurobiology that science has been attempting to solve for years. The fact that axon growth and control of its migratory movement are unrelated is an unexpected result.
“Both actions occur simultaneously and both are dependent on microtubules. And still, they are controlled independently of each other,”
said Stanislav Vinopal.
Microtubule Generation
The researchers created novel molecular tools for their study. These molecular tools enabled them to precisely control the centrosome’s ability to generate microtubules. Its activity can thus be reduced or increased.
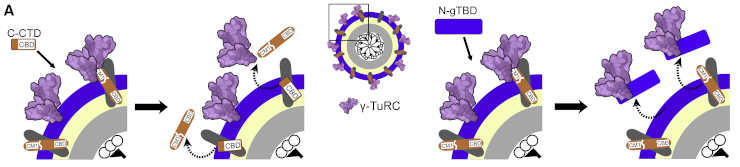
Credit: Stanislav Vinopal, Sebastian Dupraz et al. CC-BY
The researchers demonstrated that axons form independently of centrosomal activity in mouse brains. Neuronal migration, on the other hand, is greatly influenced.
“A different mechanism is apparently responsible for the growth of the axon, the so-called acentrosomal formation of microtubules. This will now become the subject of our future research,”
said Sebastian Dupraz.
Future Molecular Therapies
With their work, the scientists have been able to reconcile two previously contradictory theories: There were supporters and detractors of the theory that the centrosome plays an important role in neuronal development.
“For our study, we disentangled the two mechanisms that occur in neurons simultaneously,” says Stanislav Vinopal. “For the growth of the axon itself, we found that the centrosome is not necessary. For the process of neuronal migration, however, it plays a major role.”
During the migratory phase, the microtubule nucleating factor -tubulin was reduced at neuronal centrosomes. Because distinct microtubule networks drive neuronal polarization and radial migration, this study sheds light on how neuronal migratory defects occur in human developmental cortical dysgeneses caused by -tubulin mutations without affecting axonal tracts significantly.
The DZNE scientists’ discovery could aid in the development of a molecular therapy for some inherited diseases, such as developmental pachygyrias, that are caused by mutations in the centrosomal protein gamma-tubulin. Axons are mostly intact in these disease phenotypes, but neuronal migration is impaired.
“Presumably, the same molecular mechanism is behind these disorders, so a future therapy might focus on this point,”
the researchers said.
Reference:
- Stanislav Vinopal, Sebastian Dupraz et al. Centrosomal Microtubule Nucleation Regulates Radial Migration of Projection Neurons Independently of Polarization in the Developing Brain, Neuron February 15, 2023