By identifying two cytokines, key factors in the recruitment of blood cells essential to the formation of new blood vessels, and above all, by deciphering how these factors interact simultaneously with blood vessels, researchers at the University of Geneva (UNIGE) have highlighted an additional method of controlling tumour progression.
The results suggest that the combined use of existing drugs or under development could significantly increase their efficacy.
For a tumour to grow, it must develop blood vessels that supply nutrients and oxygen. Preventing tumour vascularization is therefore an attractive anti-tumour therapy that has been explored over the last ten years.
“Our laboratory is specialized in deciphering the molecular mechanisms involved in the recruitment of white blood cells. We have therefore taken a close look at these recruitment mechanisms in order to identify their shortcomings,”
explained Dr. Adama Sidibé, a researcher at the Faculty of Medicine at UNIGE and the first author of this work.
Endothelial Cells And Monocytes
Endothelial cells form the innermost layer of the blood vessels – the endothelium – and have the function of containing blood inside the vessels while allowing the passage of nutrients to tissues. When a new vessel is needed, the endothelial cells direct the process by allowing the recruitment of blood cells required for neovascularization, commonly called angiogenesis.
Monocytes, on the other hand, are part of the white blood cells circulating in the blood. Key elements of the immune system, they also have the ability to turn into several cell types after having passed the endothelial barrier, to fulfil different functions depending on the tissue.
A particular subpopulation of monocytes has angiogenic properties that enable vascularization. In the case of cancer, the tumour must therefore recruit these monocytes to vascularize. But how this process works has remained unclear.
Preventing Tumour Vascularization
One of the first treatments of this type, developed as early as 2004, specifically aims at slowing the growth of new blood vessels and preventing neovascularization of tumours. It is an inhibitor of a vascular endothelial growth factor, a cytokine called VEGF.
Although this drug is still part of the pharmaceutical arsenal against cancer, it has many undesirable side effects, such as high blood pressure or kidney failure, and is rapidly losing its efficacy.
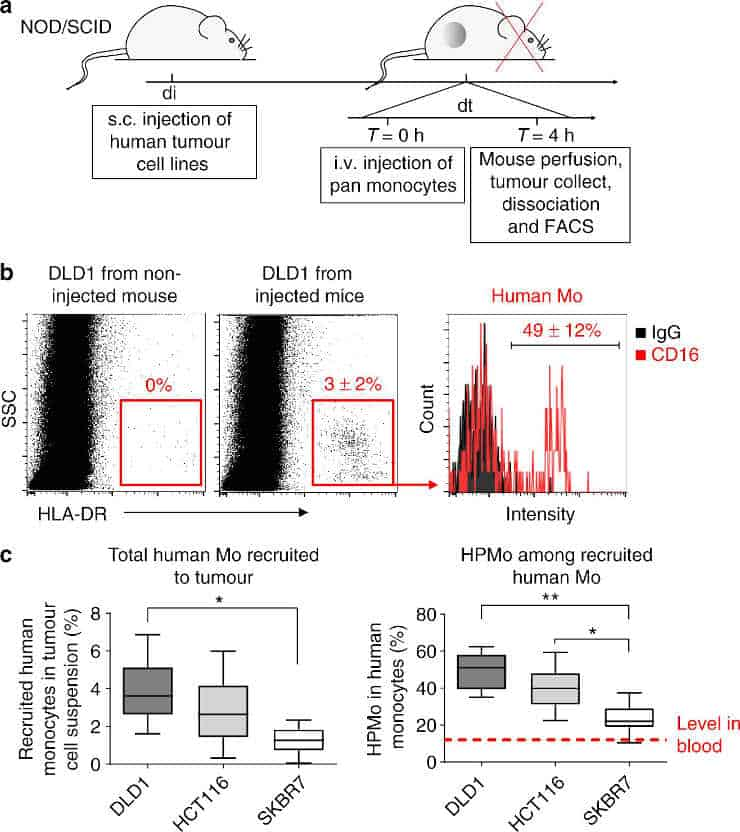
a) Time course protocol of the assay of human monocyte recruitment to human tumour xenograft (colorectal adenocarcinoma DLD1, colorectal carcinoma HCT116 and breast carcinoma SKBR7) in vivo.
Human tumour xenografts were injected subcutaneously at day di and let grow to reach 0.5 cm3 of tumour volume. The mice were then i.v. injected at day dt with freshly isolated human monocytes.
After 4 h mice were perfused with PBS-EDTA and killed to collect tumours that were then dissociated for FACS analysis of recruited human monocytes.
b) FACS analysis of recruited human proangiogenic monocytes (HLA-DR+CD16+).
Human HLA-DR staining identified human monocytes recruited to DLD1 xenograft. Gating on HLA-DR+ cells allowed quantification of human proangiogenic monocytes (CD16+).
c) Quantification of the percentage of total human monocytes within tumour cell suspension (left) and percentage of HPMo within recruited human monocytes (n = 12 tumours from six mice per group injected with monocytes from the same donor in each experiment, the data were combined from two independent experiments).
About 20–60% of recruited human monocytes were from proangiogenic subset with the highest proportion for xenografts of colorectal adenocarcinoma cells DLD1 and the lowest for xenografts of breast carcinoma SKBR7.
The data are presented as boxplot with median (horizontal line in boxes), 25–75th percentiles (box limits) and Min–Max indicated by whiskers. *p < 0.05, **p < 0.001 in one-way ANOVA
Credit: Adama Sidibe et al. CC-BY
Other strategies aimed at monocytes as a whole were also developed, with limited effectiveness. There are indeed several sub-populations of monocytes, each with a different role.
This is why a massive and indiscriminate attack unbalances the entire system, with an important consequence. When treatment ceases, the first monocytes to regain control are those that help tumours to develop.
“Therefore, knowing in detail the recruitment mechanisms of the monocyte subpopulation in charge of neovascularisation makes it possible to prevent their recruitment without disturbing the rest of the system,”
said Beat Imhof, professor at the Faculty of Medicine of UNIGE, who directed this work.
Complementary Cytokines
At first, the Geneva researchers observed the vascularisation processes of human tumour cells from different cell lines. Indeed, not all tumours have the same aggressiveness, nor the same angiogenic capacity; it was, therefore a question of observing whether the recruitment of these cells took place differently, depending on the type of malignant cells involved. Indeed, scientists have found a difference in their ability to recruit pro-angiogenic monocytes.
Dr. Sidibé described how:
“The ability of tumours to recruit angiogenic monocytes is due to the ability of the tumours themselves to produce cytokines. Tumours that preferentially recruit these monocytes secrete a number of cytokines, including TNF-alpha and VEGF.”
In the second part of their work, they used primary tumour cells taken directly from 27 patients, the scientists again identified the same cytokines.
Third step: the researchers tested separately, in an in vitro cell recruitment model, the ten cytokines identified in this study. TNF-alpha and VEGF have proven particularly effective: TNF-alpha allows adhesion to endothelial cells, while VEGF authorizes the passage through the endothelial barrier. Both are essential for angiogenesis.
The combined effect of TNF-alpha and VEGF, therefore, allows the efficient recruitment of pro-angiogenic monocytes essential for tumour vascularization.
“Our study shows that we need to target the right cytokines at the right time, and above all that we need to use the mechanisms we have discovered to define new lines of anticancer treatments. Thus, combining medicines that already exist –against VEGF, in particula– or whose development is already well advanced, would make it possible to optimise their effectiveness, rather than using them separately,”
concluded Professor Imhof. The work was supported by the Swiss National Science Foundation and the Swiss Cancer Organization (Oncosuisse).
Reference:
- Adama Sidibe, Patricia Ropraz, Stéphane Jemelin, Yalin Emre, Marine Poittevin, Marc Pocard, Paul F. Bradfield & Beat A. Imhof. Angiogenic factor-driven inflammation promotes extravasation of human proangiogenic monocytes to tumours. Nature Communications, Volume 9, Article number: 355 (2018) doi:10.1038/s41467-017-02610-0
Last Updated on January 7, 2023