A large study assessing the risks of developing dementia associated with different types and durations of menopausal hormone therapy found no increased risk regardless of hormone type, dose, or duration1.
Within the subgroup of women with a specific diagnosis for Alzheimer’s disease, a slight increasing risk association was found with use of estrogen-progestogen treatments, but measurable only for long-term usage (5 years or more).
The work brings clarity to previously inconsistent findings and should reassure women in need of menopausal hormone replacement therapy.
Previous HRT Studies
Hormone replacement therapy (HRT), also known as menopausal hormone therapy (MHT), is used to relieve menopausal symptoms such as hot flashes, sleep disturbance, mood swings, memory losses and depression. Treatments include tablets containing estrogen only, or a combination of estrogen and progestogen, as well as patches, gels and creams.
Certain menopausal symptoms are similar to early signs of dementia. Laboratory studies and small trials have suggested a beneficial link between estrogen and age related brain decline. However, the largest trial of MHT, the Women’s Health Initiative Memory Study, found an increased risk of developing dementia among users of estrogen-progestogen treatments2.
Additionally, a recent large observational study in Finland flagged an increased risk of developing Alzheimer’s disease among users of both estrogen-only and estrogen-progestogen treatments, but the study3 had some methodological weaknesses.
Primary Care Databases
To address this uncertainty, researchers at the Universities of Nottingham, Oxford and Southampton used two UK primary care databases (QResearch and CPRD) to analyse MHT prescriptions for the 118,501 women aged 55 and older diagnosed with dementia between 1998 and 2020 (cases), and 497,416 women matched by age and general practice, but with no records for dementia (controls).
All information from MHT prescriptions issued more than three years before the case diagnosis was used, including hormone type, dose, and method of administration.
Other relevant factors, such as family history, smoking, alcohol consumption, pre-existing conditions (comorbidities), and other prescribed drugs were taken into account in the analysis.
Overall, 16,291 (14%) cases and 68,726 (14%) controls had been exposed to menopausal hormone therapy in the period up to three years before diagnosis.
No Increased Risks Overall
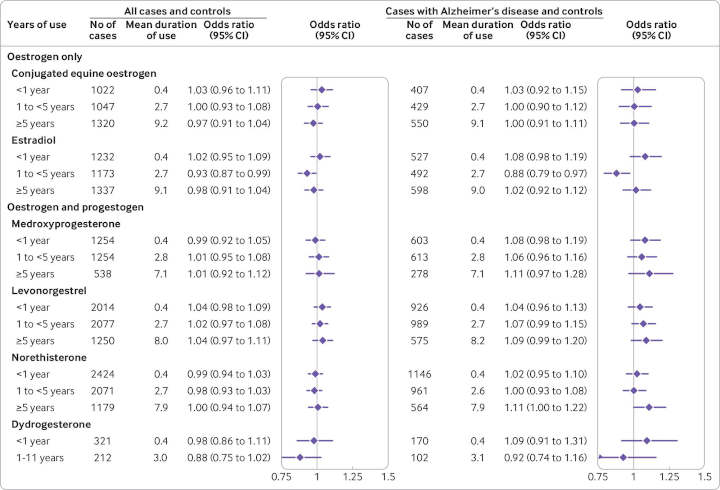
After adjusting for the full range of potentially confounding factors, the researchers found no overall associations between use of hormone therapy and risk of dementia, regardless of hormone type, application, dose, or duration of treatment.
Within the subgroup of younger than 80 years who had been taking estrogen-only therapy for 10 years or more, they found a slightly decreased risk of dementia.
However, an analysis of cases with a specific diagnosis of Alzheimer’s disease showed a slight increase in risk associated with estrogen-progestogen therapy. This rose gradually with each year of exposure, reaching an average 11% increased risk for use of between 5 and 9 years and an average 19% for use of 10 years or more — equivalent to, respectively, five and seven extra cases per 10,000 woman years.
Study Limitations
This is an observational study, as such it cannot establish any causal link, and the researchers acknowledge some limitations, such as incomplete recording of menopausal symptoms, particularly for women registered after their menopause, that may have affected their results.
However, the study used a large data sample from primary care records and was designed not only to assess overall risk for women exposed to different types of long term hormone therapy but also to explore the differences between component hormones, offering new, more reliable estimates for doctors and their patients.
The researchers say this study provides the most detailed estimates of risk for individual treatments, and their results are in line with existing concerns in guidelines about long term exposures to combined hormone therapy treatments.
These observations do not change the recommendation that menopausal hormone therapy should not be used to prevent dementia, say US researchers in an accompanying opinion article. At the same time, it is helpful for providers to put dementia findings in context for patients, they add.
- Vinogradova Y, Dening T, Hippisley-Cox J, Taylor L, Moore M, Coupland C et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPR databases. BMJ 2021; 374 :n2182 doi:10.1136/bmj.n2182 ↩︎
- Shumaker SA, Legault C, Rapp SR, et al., WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289: 2651-62. doi:10.1001/jama.289.20.2651 pmid:12771112 ↩︎
- Savolainen-Peltonen H, Rahkola-Soisalo P, Hoti F, et al. Use of postmenopausal hormone therapy and risk of Alzheimer’s disease in Finland: nationwide case-control study. BMJ2019;364:l665. doi:10.1136/bmj.l665 pmid:30842086 ↩︎
Last Updated on November 29, 2023