A research team from Johns Hopkins University in the USA has recreated human brain microvessels within the lab.
This is important as replicating brain-microvessels and the blood-brain barrier is crucial to studying both healthy function of brain blood vessels and the function and mechanisms of this in disease. Multiple diseases affect the blood vessels of the brain, with Alzheimer’s disease, multiple sclerosis and vascular dementia being but a few examples of this.
To build the brain-microvessel the researchers had to initially investigate which conditions would allow for functional vascular growth. This led to the group utilising different proteins and molecules which natively surround the cells in our brains, in combination and alone, to find ideal conditions for promoting the functional growth of the microvessels.
Extracellular Environment Interaction
This approach utilises the response of brain microvascular cells to their extracellular environment, and their critical interactions with the extracellular matrix (ECM) as part of the neurovascular unit.
The ECM physically interacts with neurovascular cells to enable for mechanical feedback of the ECM. This mechanical feedback is crucial as previous studies have shown that cells react differently when grown in different stiffness environments; another factor which aids growth of bone cells in their stiff environment compared to neurons in the soft brain.
The authors highlight the importance of replicating the ECM within a blood-brain barrier model, as it is the component of the brain and vasculature which allows for structural support and chemical cues to maintain function. To mimic this they trialled different natural materials present in the ECM which are also used commonly for tissue engineering (hyaluronan, fibrin and collagen I).
From this they found that collagen I based hydrogels were the ideal material to move forwards with for experiments, as the hyaluronan did not allow for cell attachment and the fibrin was quickly degraded by the brain-endothelial iPSC cells used. Benefits of using collagen I included cell adhesion, stability and a modifiable environment for cells.
Although collagen I is not actually found in the brain, it provides the appropriate physical cues and binding sites necessary for neurovascular culture.
Collagen Hydrogel Stiffness
The team found that increasing the stiffness of collagen I hydrogels (189, 1840, and 3800 Pa) increased the coverage and binding of the cells across the entire surface area, with lower stiffness correlating to clumping of cells rather than a uniform distribution. Previous experiments have evaluated the effects of substrate stiffness on endothelial monolayer integrity but have only investigated this with 1.1 kPa, 40 kPa, and 50 GPa stiffness materials.
To further replicate the brain vasculature, the group coated the collagen I hydrogels with different components of the immediate surrounding ECM of the brain blood vessels (the basement membrane).
They used collagen IV (the native collagen of the brain), fibronectin, laminin, agrin, and perlecan to coat the collagen I gels, and found that in every case coating aided cell spreading with no difference being found from coating with one, two or three different components.
The optimal coating material(s) was found to be agrin or a combination of collagen IV and fibronectin, with both options resulting in functional monolayer production.
Brain-specific Microvessel
After investigating the matrix compositions suitable for effective brain-specific endothelial monolayer formation, the group then used this information to build a full brain-specific microvessel (figure 1).
The researchers fabricated a collagen I hydrogel block with a rod running through the middle, this enabled the creation of a central channel suitable for coating with the natural matrix components established from the monolayer experiments, with subsequent seeding of the brain-endothelial cells to the coated walls of the channel.
The group were able to image the microvessel and establish that the endothelial cells are able to bind and form functional inter-cellular junctions.
Additionally, the group tested the permeability of the microvessels by running a dye of a known molecular weight (Lucifer yellow) through the channel. This showed that the endothelium created in the microvessel was impermeable to the dye, and indicated that functional inter-cellular junctions had formed.
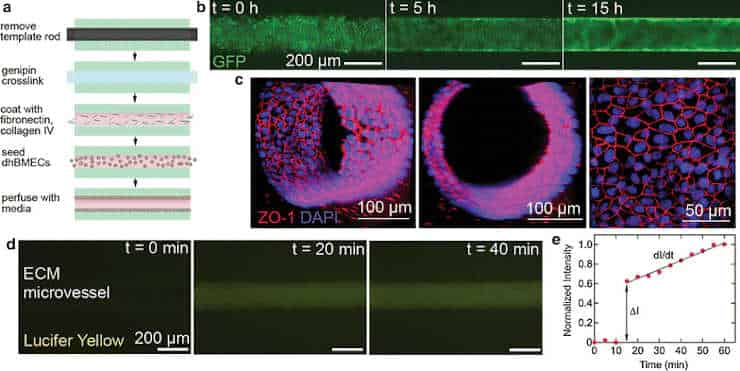
(a) Schematic of microvessel production.
(b) Endothelial cells in green showing adherence to the microvessel walls.
(c) Junction staining of endothelial cell walls (red) and cell nuclei (blue).
(d) Time course of Lucifer yellow seeding, showing the impermeability of the cell wall.
Reproduced from BMC publication Katt et al., 2018, CC BY 4.0
The researchers used a type of reprogrammed stem cell which can be grown into multiple cell types of the body.
This stem cell is an “induced pluripotent stem cell” (iPSC), and is reprogrammed from fibroblast cells, which can be extracted from the skin. This means they can be taken from human patients and be grown into different cell types for research purposes whilst retaining the genetic information of the patient.
This model can be used in future for multiple experimental applications. For example, by using patient-specific iPSCs to fabricate a microvessel, it is possible to study patient-specific responses to drugs or to establish the cellular and molecular mechanisms responsible for neurovascular dysfunction in neurodegenerative diseases.
In particular, the ability of drugs to pass the blood-brain barrier to elicit an effect in the treatment of Alzheimer’s disease or other neurodegenerative diseases would be useful to develop a patient-specific approach to research.
Reference:
- Moriah E. Katt, Raleigh M. Linville, Lakyn N. Mayo, Zinnia S. Xu and Peter C. Searson. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids and Barriers of the CNS. 20 February 2018. doi: 10.1186/s12987-018-0092-7
Last Updated on October 31, 2023