According to new UCLA Health research, astrocytes appear to play an important role in obsessive-compulsive disorder-related behaviours.
Researchers were surprised by the new clue about the brain mechanisms underlying OCD, a disorder that is still poorly understood. They wanted to know how neurons interact with astrocytes, the star-shaped helper cells that support and protect neurons.
Scientists are still trying to figure out how these complex cells play a role in psychiatric and neurodegenerative diseases.
Obsessive-compulsive disorder, a lifelong anxiety disorder characterized by repetitive thoughts and actions, affects an estimated 2-3% of the US population at some point in their lives, though its prevalence may be higher due to underreporting and underdiagnosis. Although psychotherapy, antidepressant medication, or both are commonly prescribed for obsessive-compulsive disorder, available treatment is ineffective for many patients.
New Cellular Mechanism
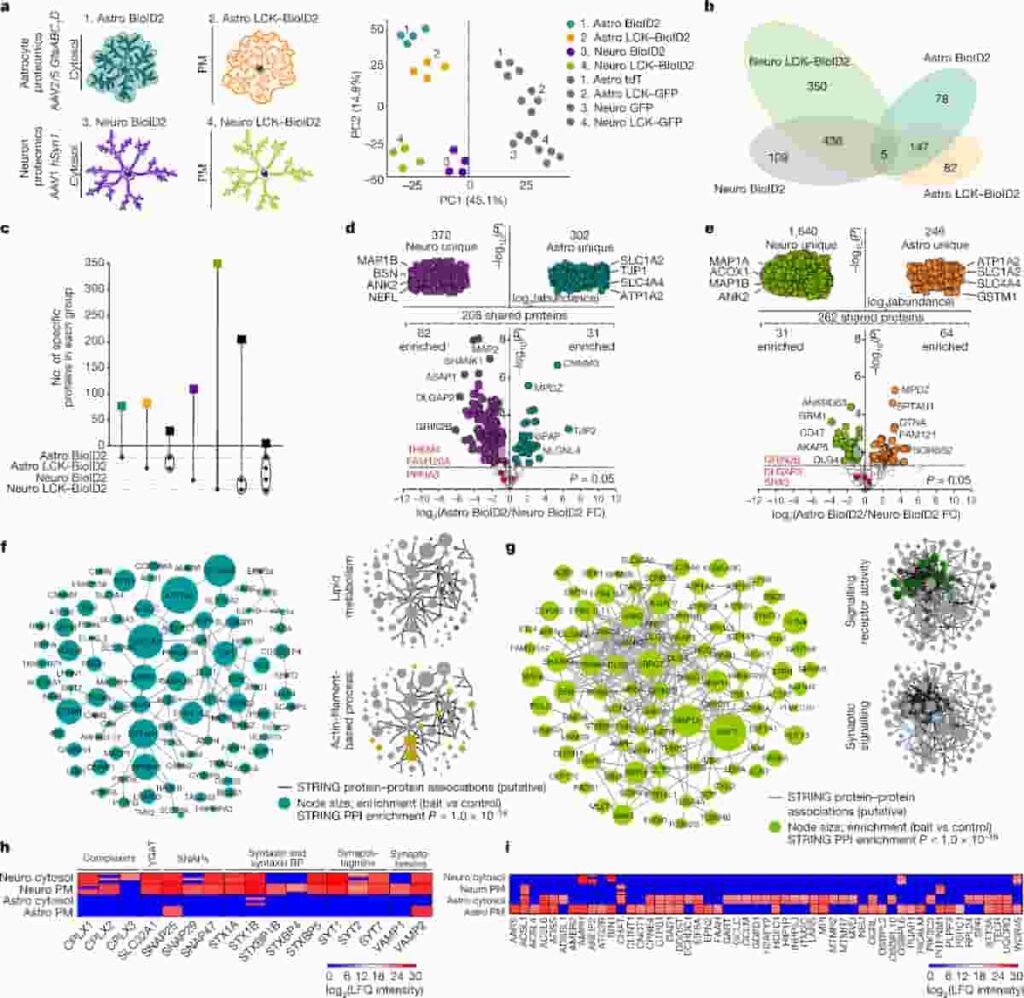
(a) Left, cartoons representing genetically targeted BioID2 in the cytosol and plasma membrane (PM) of astrocytes and neurons. Right, PCA plot of all proteins detected by mass sepctrometry. (b) Clustergram depicting the specific number of proteins detected in each BioID2 construct experiment of astrocytes and neurons. All proteins hereafter represent those that were significant. (c) UpSet plot of BioID2-identified proteins. (d) LFQ comparison of proteins detected by cytosolic Astro BioID2 and Neuro BioID2.
(e) As in d but for PM Astro BioID2 and Neuro BioID2. (f) Left, STRING analysis map of the top 100 proteins identified with Astro BioID2 and Astro LCK–BioID2. Node size represents the enrichment of each protein versus the GFP control. (g) As in f, but for Neuro BioID2 and Neuro LCK–BioID2. (h) Expression levels (LFQ intensity) of Ca2+-dependent vesicle release proteins identified by each BioID2 construct. BPs, binding proteins. (i) Expression levels of proteins related to lipid metabolism identified in each BioID2 construct.
Credit: Soto, J.S., Jami-Alahmadi, Y., Chacon, J. et al. CC-BY
The UCLA researchers discovered a protein associated with OCD and repetitive behaviours in neurons was also found in astrocytes by studying the proteins expressed by neurons and astrocytes in mice. The discovery suggests that therapeutic strategies targeting astrocytes and neurons could be beneficial for obsessive-compulsive disorder and other brain disorders.
“Our research has revealed a new cellular mechanism, which not only involves neurons, something we already knew, but also involves astrocytes, working together. Now we could expand our research in this area to cover additional mechanisms and cells,”
said corresponding author Baljit Khakh, a professor of physiology and neurobiology at the David Geffen School of Medicine at UCLA.
The striatum, a brain region involved in decision-making and motor control, is thought to play a key role in obsessive-compulsive disorder. That is precisely the area of the brain studied by UCLA researchers when they sought to investigate the interactions between astrocytes and neurons.
Protein Expression
Khakh is one of the researchers who has studied astrocytes extensively in recent years, thanks to technological advances that have made it easier to study these complex cells.
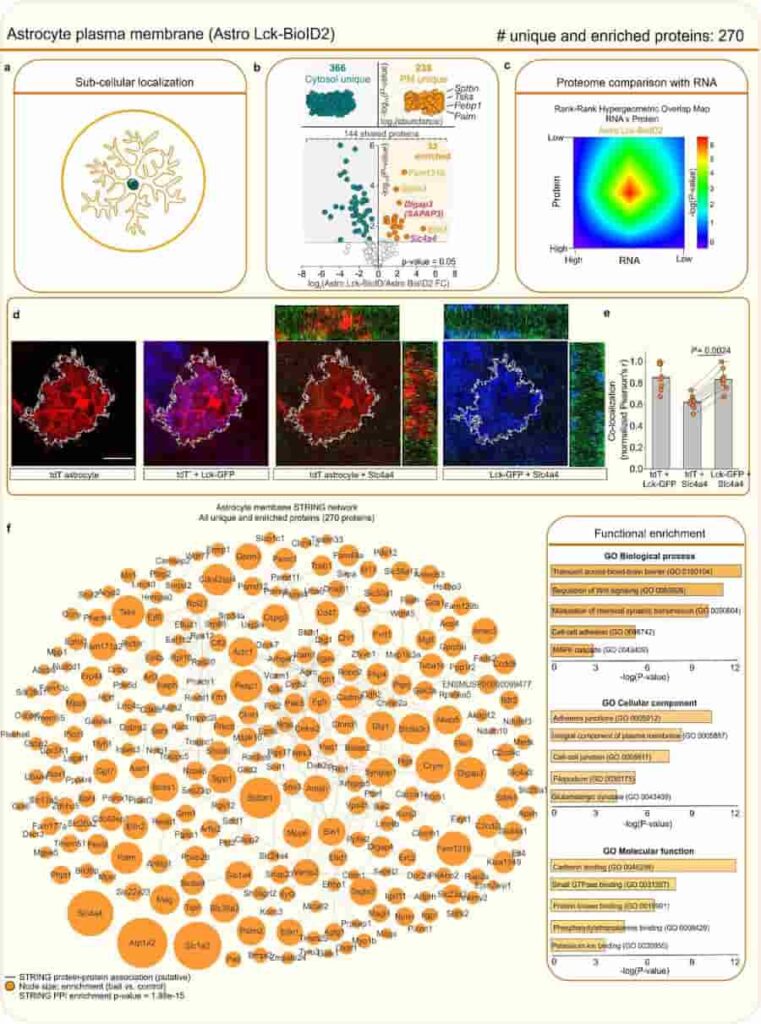
(a) BioID2 that is targeted to the plasma membrane biotinylates proteins near the plasma membrane. (b) Label-free based quantification comparison of significant proteins (Log2FC > 1 and FDR < 0.05 versus GFP controls) detected in the cytosolic Astro BioID2 and plasma membrane Astro Lck-BioID2 reveal plasma membrane enriched proteins. Top half of the volcano plot shows 238 unique Lck-BioID2 proteins when compared to cytosol. The top four most abundant proteins for Lck-BioID2 are shown. Lower half of volcano plot shows comparison of 144 proteins that were common in both cytosolic BioID2 and Lck-BioID2. The five highest enriched proteins for Lck-BioID2 (Log2FC > 2) are shown. Magenta label shows protein that was validated with IHC in panel d. Red label shows Dlgap3/SAPAP3 is enriched in the astrocyte plasma membrane. (c) Heat map shows the rank-rank hypergeometric overlap (RRHO) of the RNA and protein rank for the 270 plasma membrane proteins. Each pixel represents the significance of overlap between the two datasets in –log10(P-value). Red pixels represent highly significant overlap. Color scale denotes the range of P-values at the negative log10 scale (bin size = 100). (d) IHC analysis of Slc4a4 (Nbc1) protein in tdTomato and Lck-GFP labeled astrocytes shows co-localization within the astrocyte territory. Scale bar represents 20 μm. (e) Co-localization analysis using Pearson’s r co-efficient shows high co-localization between Lck-GFP and Slc4a4 (Nbc1). The mean and SEM are shown (n = 8 tdTomato+ cells from 4 mice; Two-tailed paired t-test). (f) Scale-free STRING analysis protein-protein association map of the 270 unique and enriched biotinylated proteins identified in astrocyte plasma membrane with Astro Lck-BioID2 . Node size represents the enrichment of each protein vs the GFP control (log2(BioID2/GFP)). Edges represent putative interactions from the STRING database. Bar graphs show the functional enrichment analysis of the 270 proteins using “Biological process”, “Cellular component”, and “Molecular function” terms from Enrichr.
Credit: Soto, J.S., Jami-Alahmadi, Y., Chacon, J. et al. CC-BY
While previous research compared gene expression between neurons and astrocytes, this new study advanced our understanding of the two cell types’ interactions by analyzing protein expression.
“We really have to look at the proteins because they are very complex and diverse. Depending on which cell expresses which proteins, we can predict the functions of that cell,”
said co-author Joselyn Soto, a neuroscience Ph.D. student at UCLA’s medical school.
Thus, they examined astrocyte and neuron cytosolic and plasma membrane compartments to learn how these cells differ at the protein level in their signalling machinery.
SAPAP3
The researchers used a variety of techniques to isolate and visualize proteins in the striatum’s neurons and astrocytes. When they compared proteins found in neurons and astrocytes, they discovered that both contained SAPAP3, a protein associated with obsessive-compulsive disorders.
SAP90/PSD95-associated protein (SAPAP) family proteins interact with other proteins to form a key scaffolding complex at excitatory glutamatergic synapses.
The study’s findings were validated by reintroducing the SAPAP3 protein into neurons and astrocytes of mice that had been genetically modified to lack the gene responsible for protein production. When the researchers measured the protein’s effects on compulsion and anxiety, two of the typical hallmarks of obsessive-compulsive disorder, they discovered that the two types of cells interacted differently.
After the SAPAP3 protein was returned to astrocytes and neurons, the mice no longer groomed themselves compulsively, implying that both types of cells could be viable targets for treatments to reduce compulsion.
However, only neurons expressing the SAPAP3 protein were linked to decreased anxiety in mice, implying that astrocytes would not be a good target for anxiety treatments in obsessive-compulsive disorder.
Important Pathophysiology Implications
Soto stated that future research would delve deeper into how these cells’ interactions affect behaviour.
“These are both major cell types, one doesn’t work without the other,” he said. “We really wanted to understand how these multicellular interactions within this brain region give rise to these complex behaviours, including compulsion and anxiety.”
Adding that more research is needed to understand how astrocytes are formed and maintained, Khakh stated that the unexpected findings of this new study demonstrated the importance of pursuing basic biology questions to help form new ideas about the causes of diseases.
“The finding that many proteins were preferentially enriched in astrocyte subproteomes has important implications for understanding pathophysiology during neurodegeneration, injury, stroke, trauma and addiction that are accompanied with altered astrocyte morphology and signalling,”
the authors write.
Reference:
- Soto, J.S., Jami-Alahmadi, Y., Chacon, J. et al. Astrocyte–neuron subproteomes and obsessive–compulsive disorder mechanisms. Nature (2023)
Last Updated on September 20, 2023