Newly published research, from a group based in Wisconsin, suggests a link between the bacterial content of the gut with the onset of Alzheimer’s disease (AD). Although the link has been made in animal models previously, this is the first time the ‘microbiome’ (a person’s full gut bacterial profile) has been substantially characterised in humans.
The group found that AD patients had a distinct composition of bacteria with reduced range of bacterial diversity compared to age and sex matched counterparts. These results also correlated with cerebrospinal fluid markers of AD taken from the same patients.
Non-Invasive Markers
This study utilized the analysis of faecal samples from 25 Alzheimer’s patients and 25 age-matched controls to characterise the bacterial contents of the microbiome. This allowed for a non-invasive assessment of disease risk factors and may offer an early diagnostic and prognostic marker for the disease.
The bacterial content of the gut is established from an early age and remains relatively stable throughout life. Although the microbiome is stable, there are vast differences in bacterial composition from person to person, which could be useful in attributing certain bacteria to Alzheimer’s disease in future follow up studies.
Complex Microbiome
The gut microbiome content is extremely complex, harbouring more bacteria than there are actual cells in the body.
Although the microbiome may seem to be an obvious player in diseases like irritable bowel disease, it also has key roles in the synthesis of vitamins, priming of the immune system and protection against pathogen outgrowth.
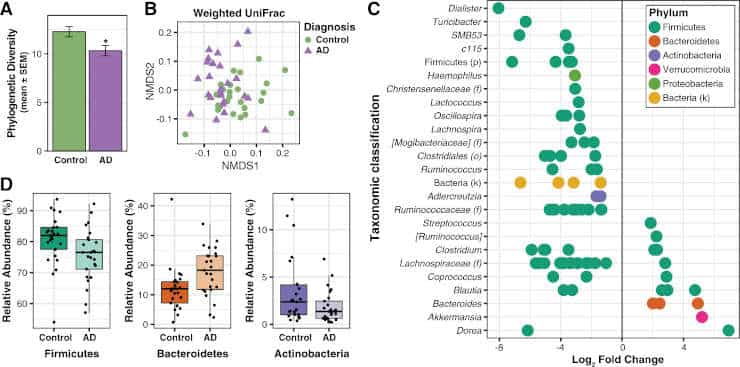
(A) Faith’s
Phylogenetic Diversity is decreased in the microbiome of AD participants. *p < 0.05.
(B) Non-metric
multidimensional scaling (NMDS) plot of weighted UniFrac analysis of relative sample OTU composition.
NMDS analysis was limited to two dimensions, with a stress measurement of 0.17. Each dot represents a
scaled measure of the composition of a given participant, color- and shape-coded by cohort.
(C) Differential
abundance analysis identified 14 OTUs that were increased and 68 OTUs that were decreased in AD relative
to Control participants (p < 0.05, FDR-corrected). Each point represents an OTU.
Data plotted as log2 fold
change; OTUs to the right of the zero line are more abundant and OTUs to the left of the zero line are less
abundant in AD compared to Control groups. OTUs are organized on the y-axis according to the lowest
taxonomic classification possible.
(D) OTUs grouped at the phylum level and analyzed using Metastats show
that AD participants have decreased abundance of Firmicutes and Actinobacteria, and increased abundance of
Bacteroidetes compared to Control participants (p < 0.05, FDR-corrected).
Tukey plots show median, IQR, and
participant data points for phylum relative abundance.
Credit: Vogt NM, et al. Republished via Creative Commons
The decreased microbiome diversity discovered in AD patients in this study correlates with findings for multiple other diseases; including irritable bowel disease, diabetes and Parkinson’s disease. In fact, one of the reduced bacteria classes linked to onset of AD in this study, Firmicutes, has also been linked to the onset of both obesity and type-II diabetes.
Paving The Way Forward
Previous research has paved the way for this study, with animal models indicating that the gut bacterial content may affect the production of amyloid, the protein plaque hallmark of AD. Additionally, a previous human study has shown increased pro-inflammatory and decreased anti-inflammatory levels of bacteria in Alzheimer’s disease patients’ microbiota.
Collectively, these findings suggest that there has been a distinct lack of investigations into the role of gut bacteria in AD. The authors have outlined the future for their research, with additional animal experiments and a longitudinal study with human samples being crucial to establishing the true effect of the microbiome on AD onset and progression.
The current work could subsequently lead to targeting the microbiome therapeutically in order to treat, or better yet, prevent the onset of the disease. This could potentially be achieved by finding ways to increase the bacteria missing from AD patients or even from deciphering the metabolites which these bacteria produce.
Reference:
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer’s disease. Scientific Reports: 19 October 2017. doi:10.1038/s41598-017-13601-y
Last Updated on April 9, 2024